From Wikipedia, the free encyclopedia
Chemical compound
Fluorometholone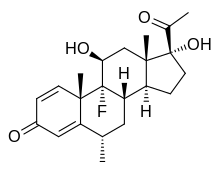 |
|
| Other names | (6S,8S,9R,10S,11S,13S,14S,17R)-17-acetyl-9-fluoro-11,17-dihydroxy-6,10,13-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one |
|---|
| AHFS/Drugs.com | Monograph |
|---|
| MedlinePlus | a682660 |
|---|
| ATC code | |
|---|
|
| Legal status |
|
|---|
|
(1R,2S,8S,10S,11S,14R,15S,17S)-14-acetyl-1-fluoro-14,17-dihydroxy-2,8,15-trimethyltetracyclo[8.7.0.02,7.011,15]heptadeca-3,6-dien-5-one
|
| CAS Number | |
|---|
| PubChem CID | |
|---|
| IUPHAR/BPS | |
|---|
| DrugBank | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| KEGG | |
|---|
| ChEBI | |
|---|
| ChEMBL | |
|---|
| CompTox Dashboard (EPA) | |
|---|
| ECHA InfoCard | 100.006.402  |
|---|
|
| Formula | C22H29FO4 |
|---|
| Molar mass | 376.468 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
O=C(C)[C@]3(O)[C@]2(C[C@H](O)[C@]4(F)[C@@]/1(\C(=C/C(=O)\C=C\1)[C@@H](C)C[C@H]4[C@@H]2CC3)C)C
|
InChI=1S/C22H29FO4/c1-12-9-17-15-6-8-21(27,13(2)24)20(15,4)11-18(26)22(17,23)19(3)7-5-14(25)10-16(12)19/h5,7,10,12,15,17-18,26-27H,6,8-9,11H2,1-4H3/t12-,15-,17-,18-,19-,20-,21-,22-/m0/s1  Y YKey:FAOZLTXFLGPHNG-KNAQIMQKSA-N  Y Y
|
 N N Y (what is this?) (verify) Y (what is this?) (verify) |
Fluorometholone (INN, BAN, JAN) (brand names Efflumidex, Flucon, FML Forte, FML, others), also known as 6α-methyl-9α-fluoro-11β,17α-dihydroxypregna-1,4-diene-3,20-dione, is a synthetic glucocorticoid which is used in the treatment of inflammatory eye diseases.[2][3][4] The C17α acetate ester, fluorometholone acetate (brand name Flarex), is also a glucocorticoid and is used for similar indications.[2][3][4]
|
|---|
| GRTooltip Glucocorticoid receptor | |
|---|
|
|
|---|
| PRTooltip Progesterone receptor | | Agonists |
- Testosterone derivatives: Progestins: 6,6-Difluoronorethisterone
- 6,6-Difluoronorethisterone acetate
- 17α-Allyl-19-nortestosterone
- Allylestrenol
- Altrenogest
- Chloroethynylnorgestrel
- Cingestol
- Danazol
- Desogestrel
- Dienogest
- Ethinylandrostenediol
- Ethisterone
- Ethynerone
- Etonogestrel
- Etynodiol
- Etynodiol diacetate
- Gestodene
- Gestrinone
- Levonorgestrel
- Levonorgestrel esters (e.g., levonorgestrel butanoate)
- Lynestrenol
- Lynestrenol phenylpropionate
- Metynodiol
- Metynodiol diacetate
- Norelgestromin
- Norethisterone (norethindrone)
- Norethisterone esters (e.g., norethisterone acetate, norethisterone enanthate)
- Noretynodrel
- Norgesterone
- Norgestimate
- Norgestrel
- Norgestrienone
- Norvinisterone
- Oxendolone
- Quingestanol
- Quingestanol acetate
- Tibolone
- Tigestol
- Tosagestin; Anabolic–androgenic steroids: 11β-Methyl-19-nortestosterone
- 11β-Methyl-19-nortestosterone dodecylcarbonate
- 19-Nor-5-androstenediol
- 19-Nor-5-androstenedione
- 19-Nordehydroepiandrosterone
- Bolandiol
- Bolandiol dipropionate
- Bolandione
- Dimethisterone
- Dienedione
- Dienolone
- Dimethandrolone
- Dimethandrolone buciclate
- Dimethandrolone dodecylcarbonate
- Dimethandrolone undecanoate
- Dimethyldienolone
- Dimethyltrienolone
- Ethyldienolone
- Ethylestrenol (ethylnandrol)
- Methyldienolone
- Metribolone (R-1881)
- Methoxydienone (methoxygonadiene)
- Mibolerone
- Nandrolone
- Nandrolone esters (e.g., nandrolone decanoate, nandrolone phenylpropionate)
- Norethandrolone
- Normethandrone (methylestrenolone, normethandrolone, normethisterone)
- RU-2309
- Tetrahydrogestrinone
- Trenbolone (trienolone)
- Trenbolone esters (e.g., trenbolone acetate, trenbolone enanthate)
- Trendione
- Trestolone
- Trestolone acetate
|
|---|
Mixed
(SPRMsTooltip Selective progesterone receptor modulators) | |
|---|
| Antagonists | |
|---|
|
|---|
mPRTooltip Membrane progesterone receptor
(PAQRTooltip Progestin and adipoQ receptor) | |
|---|
|
|
|---|
| CARTooltip Constitutive androstane receptor | |
|---|
| PXRTooltip Pregnane X receptor | |
|---|
|

