Darolutamide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nubeqa |
| Other names | Darramamide, ODM-201, BAY-1841788 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619045 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Nonsteroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ≤30%[4] |
| Protein binding | Darolutamide: 92%[4] Ketodarolutamide: 99.8%[4] |
| Metabolism | Dehydrogenation (CYP3A4), glucuronidation (UGT1A9, UGT1A1)[4] |
| Metabolites | Ketodarolutamide[4][7] |
| Elimination half-life | 16–20 hours[4][7] |
| Excretion | Urine: 63.4%[4] Feces: 32.4%[4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.264.885 |
| Chemical and physical data | |
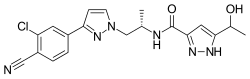
| Formula | C19H19ClN6O2 |
| Molar mass | 398.85 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Darolutamide, sold under the brand name Nubeqa, is an antiandrogen medication which is used in the treatment of non-metastatic castration-resistant prostate cancer in men.[8][4][5][9][10] It is specifically approved to treat non-metastatic castration-resistant prostate cancer (nmCRPC) in conjunction with surgical or medical castration.[4] The medication is taken by mouth twice per day with food.[4]
Side effects of darolutamide added to castration may include fatigue, asthenia, pain in the arms and legs, and rash.[4] Darolutamide is a nonsteroidal antiandrogen (NSAA), and acts as a selective antagonist of the androgen receptor (AR).[4][9][10] It has been referred to as a second- or third-generation NSAA.[11][12]
Darolutamide was patented in 2011 [13] and was approved for medical use in USA in July 2019,[8][14] in the European Union in March 2020 [5] in Australia in July 2020.[15] and in Canada in 2020,
Medical uses
[edit]Darolutamide is approved for use concurrently with a gonadotropin-releasing hormone (GnRH) agonist or antagonist or bilateral orchiectomy in the treatment of non-metastatic castration-resistant prostate cancer (nmCRPC) in men.[9][10] It is used at a dosage of 600 mg orally twice per day (1,200 mg/day total) with food.[4] In individuals with severe renal impairment or moderate hepatic impairment, darolutamide is used at a dosage of 300 mg orally twice per day (600 mg/day total) with food.[4] No dosage adjustment is needed for mild to moderate renal impairment or mild hepatic impairment, whereas appropriate dosage adjustment for end-stage kidney disease and severe hepatic impairment is unknown.[4]
Two 2020 meta-analyses reported that enzalutamide and apalutamide seemed to be more effective than darolutamide in improving metastasis-free survival (MFS), however 2021 matched adjusted indirect comparison showed no significant differences between drugs in terms of MFS.[16][17][18] According to 2021 meta-analysis darolutamide was ranked first in terms of improving overall survival (OS).[19] Also, darolutamide showed significantly lower rate of grade 3-5 adverse events (AE) compared to both enzalutamide and apalutamide.[19]
Available forms
[edit]Darolutamide is provided in the form of 300 mg oral film-coated tablets.[4]
Contraindications
[edit]Darolutamide has no contraindications in men.[4] However, the medication may have teratogenic effects in male fetuses due to its antiandrogenic effects and hence should not be used by women who are pregnant.[4]
Side effects
[edit]The most common side effects of darolutamide in clinical trials (≥2% incidence) in castrated men included fatigue and asthenia (16% vs. 11% for placebo), pain in extremities (6% vs. 3% for placebo), and rash (3% vs. 1% for placebo).[4] Darolutamide was also associated with higher incidences of ischemic heart disease (4.0% vs. 3.4% for placebo) and heart failure (2.1% vs. 0.9% for placebo).[4] In terms of laboratory test abnormalities, darolutamide was associated with decreased neutrophil count (20% vs. 9% for placebo), increased aspartate aminotransferase (AST) (23% vs. 14% for placebo; Grade 3–4: 0.5% vs. 0.2% for placebo), and increased bilirubin (16% vs. 7% for placebo).[4] In the clinical studies, 88% of patients treated with darolutamide were age 65 years or older.[4]
No seizures have been observed with darolutamide in clinical trials.[7][20] Darolutamide is an expected teratogen and has a theoretical risk of birth defects in male infants if taken by women during pregnancy.[4] It may impair male fertility.[4] When used as a monotherapy (i.e., without surgical or medical castration) in men, NSAAs are known to produce feminizing breast changes including breast tenderness and gynecomastia.[21]
Overdose
[edit]Darolutamide has been studied at a dosage of up to 1,800 mg/day in clinical trials.[4] There were no dose-limiting toxicities seen at this dosage.[4] Due to its saturable absorption and lack of acute toxicity, overdose of darolutamide is not expected to result in systemic toxicity in people with intact hepatic and renal function.[4] There is no specific antidote for overdose of darolutamide.[4] In the event of darolutamide overdose, if there is no toxicity, treatment can be continued as normal.[4] If there is suspicion of toxicity, general supportive measures should be undertaken until clinical toxicity has decreased or resolved and then treatment may be continued.[4]
Interactions
[edit]Combined P-glycoprotein and strong or moderate CYP3A4 inducers such as rifampicin may decrease blood levels of darolutamide, while combined P-glycoprotein and strong CYP3A4 inhibitors such as itraconazole may increase blood levels of darolutamide.[4] Darolutamide is an inhibitor of the breast cancer resistance protein (BCRP) transporter and can increase blood levels of substrates for BCRP protein, such as rosuvastatin, by approximately 5-fold.[4]
Pharmacology
[edit]Pharmacodynamics
[edit]Darolutamide is a second- or third-generation nonsteroidal antiandrogen (NSAA).[11][12] It acts as a selective competitive silent antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[4] Its affinity (Ki) for the AR is 11 nM and its functional inhibition (IC50) of the AR is 26 nM.[10] The major metabolite of darolutamide, ketodarolutamide, has similar antiandrogenic activity relative to darolutamide (Ki = 8 nM; IC50 = 38 nM).[4][10] In addition to its actions as an AR antagonist, darolutamide has been found to act as a silent antagonist of the progesterone receptor (PR), with approximately 1% of the potency of its AR antagonism.[4]
A dosage of darolutamide of 1,200 mg/day has been found to result in a mean decrease in prostate specific antigen (PSA) levels of more than 90% in men with prostate cancer.[4][additional citation(s) needed] The addition of darolutamide to castration has been found to decrease PSA levels by more than 50% in about 50% of men at 200 mg/day, 69% of men at 400 mg/day, 83% of men at 1,200 mg/day, and 86% of men at 1,400 mg/day.[22][10][7] In accordance with its antiandrogenic activity, darolutamide monotherapy (600 mg b.i.d.) has been found to increase testosterone levels in men with prostate cancer by 43.3% on average (range 5.7 to 144.0%), from median 413 ng/dL (range 209–1183 ng/dL) at baseline to median 595 ng/dL (range 260–1500 ng/dL), after 24 weeks of treatment.[23] For comparison, testosterone levels increased by 114.3% with enzalutamide monotherapy[23] and high-dose bicalutamide monotherapy increases testosterone levels by about 59 to 97% in men with prostate cancer.[24][25][26][27] A phase 2 clinical trial directly comparing testosterone increases with darolutamide monotherapy versus enzalutamide monotherapy is underway as of January 2024.[28][29][30]
Darolutamide shows some advantages in comparison to enzalutamide and apalutamide, two other second-generation NSAAs.[10] It has been claimed to negligibly cross the blood–brain barrier, and hence is thought to have a reduced risk of seizures and other central side effects from off-target GABAA receptor inhibition.[10] However, darolutamide monotherapy has subsequently been found to increase testosterone levels, a centrally mediated antiandrogenic action.[23] Darolutamide has been found to block the activity of all tested/well-known mutant ARs in prostate cancer, including the recently identified clinically-relevant F876L mutation that produces resistance to enzalutamide and apalutamide.[10] The medication shows higher affinity and inhibitory potency at the AR relative to enzalutamide and apalutamide in vitro (Ki = 11 nM relative to 86 nM for enzalutamide and 93 nM for apalutamide; IC50 = 26 nM relative to 219 nM for enzalutamide and 200 nM for apalutamide).[10]
Darolutamide inhibits the organic anion transporting polypeptide (OATP) transporters OATP1B1 and OATP1B3 in vitro.[4] It shows no inhibition or induction of cytochrome P450 enzymes (CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4) at clinically relevant concentrations.[31] Similarly, darolutamide shows no inhibition of a variety of other transporters (P-glycoprotein, MRP2, BSEP, OATs, OCTs, MATEs, OATP2B1, NTCP) at therapeutic concentrations.[4][32]
Pharmacokinetics
[edit]Absorption
[edit]The absolute bioavailability of darolutamide with oral administration of a single 300-mg dose without food is approximately 30%.[4] The bioavailability of darolutamide is increased by about 2- to 2.5-fold when administered with food, with a similar increase in exposure occurring for ketodarolutamide.[4] Exposure to darolutamide and ketodarolutamide increases in a nearly linear or dose-proportional manner across a dose range of 100 to 700 mg (or about 0.17- to 1.17-fold the recommended 600-mg dosage).[4] No further increase in exposure to darolutamide was observed at a dosage of darolutamide of 900 mg twice per day (or 1.5 times the recommended 600-mg dosage), indicating a saturation of absorption at doses above 700 mg.[4] Following a single 600-mg dose of darolutamide, peak levels of darolutamide occur after approximately 4 hours.[4] Steady-state levels of darolutamide occur after 2 to 5 days of continuous administration with food, during which time an approximate 2-fold accumulation in darolutamide levels occurs.[4] At steady state with 600 mg/day darolutamide, mean levels of darolutamide are 4.79 μg/mL and area-under-the-curve levels of darolutamide over time 0 to 12 hours (AUC0–12) are 52.82 h•μg/mL.[4] Total exposure to ketodarolutamide is approximately 1.7-fold that of darolutamide.[4]
Distribution
[edit]The volume of distribution of darolutamide with intravenous administration is 119 L.[4] The plasma protein binding of darolutamide is 92%, with 8% circulating freely, and of ketodarolutamide is 99.8%, with 0.2% circulating unbound.[4] As such, free levels of darolutamide in the circulation are about 40-fold higher than those of ketodarolutamide.[4] Both darolutamide and ketodarolutamide are bound mainly to albumin.[4] Darolutamide and ketodarolutamide has been claimed to negligibly cross the blood–brain barrier both in mice and humans.[10] However, a subsequent study of darolutamide monotherapy in men with prostate cancer found that it increased testosterone levels, a centrally mediated antiandrogenic action.[23] Darolutamide is a known substrate of P-glycoprotein and the breast cancer resistance protein (BCRP).[33][34][35] P-Glycoprotein is known to play a major role in excluding drugs from the brain due to efflux back across the blood–brain barrier.[36][37]
Metabolism
[edit]Darolutamide is primarily metabolized into ketodarolutamide via dehydrogenation by CYP3A4 in the liver.[4] The medication is also conjugated via glucuronidation by UGT1A9 and UGT1A1.[4] The elimination half-life of darolutamide and ketodarolutamide has been reported to be approximately 20 hours.[4] A clinical study found that the elimination half-lives of darolutamide and ketodarolutamide at steady-state were 15.8 hours and 10.0 hours, respectively, with these half-lives being independent of dosage across a dose range of darolutamide of 200 to 1,800 mg/day.[7] The elimination half-life of darolutamide is far shorter than that of enzalutamide (e.g., 1.6 hours vs. 18.3 hours in mice).[10] The clearance of darolutamide following intravenous administration is 116 mL/min.[4]
Excretion
[edit]After a single oral dose of darolutamide, more than 95% of the dose is excreted in urine and feces within one week following administration.[4] A total of 63.4% darolutamide-related material is recovered in urine (about 7% as unchanged darolutamide) and a total of 32.4% darolutamide-related material (about 30% as unchanged darolutamide) is recovered in feces.[4]
Variability
[edit]No clinically significant differences in the pharmacokinetics of darolutamide have been observed in men with nmCRPC on the basis of age (48 to 95 years), race (white, Asian, black), mild-to-moderate renal impairment, or mild hepatic impairment.[4] In non-nmCRPC individuals with severe renal impairment not on dialysis, exposure to darolutamide was increased by about 2.5-fold relative to healthy people.[4] In non-nmCRPC individuals with moderate hepatic impairment, darolutamide exposure was increased by about 1.9-fold compared to healthy controls.[4] The pharmacokinetics of darolutamide have not been assessed in end-stage kidney disease or severe hepatic impairment.[4]
Chemistry
[edit]Darolutamide is a nonsteroidal compound and is structurally distinct from other marketed NSAAs, including enzalutamide and apalutamide.[10]
History
[edit]Darolutamide was developed by Orion Corporation and Bayer HealthCare.[38] Orion Corporation applied for a patent on darolutamide in October 2010, and this patent was published in May 2011.[13] Darolutamide entered phase I clinical trials in April 2011,[39] with the results of the first clinical study of darolutamide initially published in 2012.[40] The U.S. Food and Drug Administration (FDA) approved darolutamide in July 2019, under the agency's priority review designation.[8]
Approval was based on ARAMIS,[41] a multicenter, double-blind, placebo-controlled clinical trial in 1,509 patients with non-metastatic castration resistant prostate cancer. Patients were randomized (2:1) to receive either 600 mg darolutamide orally twice daily (n=955) or matching placebo (n=554). All patients received a gonadotropin-releasing hormone (GnRH) analog concurrently or had a previous bilateral orchiectomy. Twelve patients with previous seizure histories were treated on the darolutamide arm.[8][14]
The primary endpoint was metastasis free survival (MFS), defined as the time from randomization to first evidence of distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first. The median MFS was 40.4 months (95% CI: 34.3, not reached) for patients treated with darolutamide compared with 18.4 months (95% CI: 15.5, 22.3) for those receiving placebo (hazard ratio 0.41; 95% CI: 0.34, 0.50; p<0.0001).
Darolutamide was associated with greater benefits than placebo for all secondary end points, including overall survival (hazard ratio 0.69; 95% CI: 0.53-0.88; P=0.003), time to pain progression (median 40.3 months vs. 25.4 months; hazard ratio 0.65; 95% CI: 0.53-0.79; P<0.001), time to cytotoxic chemotherapy (hazard ratio 0.43; 95% CI: 0.31-0.60), and time to a symptomatic skeletal event (hazard ratio 0.43; 95% CI: 0.22-0.84).[41]
Society and culture
[edit]Generic names
[edit]Darolutamide is the generic name of the medication and its INN and USAN.[42] It is also known by its developmental code names ODM-201 and BAY-1841788.[38]
Brand names
[edit]Darolutamide is marketed under the brand name Nubeqa.[4][8][5]
Availability
[edit]Darolutamide is available in the United States, Canada and the European Union.[4][8][5][43]
Research
[edit]Darolutamide monotherapy is being studied in comparison to androgen deprivation therapy with GnRH agonist or antagonist monotherapy in men with treatment-naive prostate cancer.[22][44] As of 2018, it is entering a phase II clinical trial for this indication.[22][44] In 2020, completion of this study had been expected in 2021 or 2022.[45]
Darolutamide is being studied for the treatment of breast cancer in women.[38] As of November 2019, it is in phase II clinical trials for this indication.[38]
References
[edit]- ^ "Nubeqa Australian prescription medicine decision summary". Therapeutic Goods Administration (TGA). 4 March 2020. Retrieved 16 August 2020.
- ^ "Product Monograph" (PDF). hres.ca. Retrieved 25 October 2023.
- ^ "Summary Basis of Decision (SBD) for Nubeqa". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl "Nubeqa- darolutamide tablet, film coated". DailyMed. 31 July 2019. Retrieved 22 November 2019.
- ^ a b c d e "Nubeqa EPAR". European Medicines Agency (EMA). 29 January 2020. Retrieved 16 August 2020.
- ^ "Nubeqa Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ a b c d e Fizazi K, Massard C, Bono P, Jones R, Kataja V, James N, et al. (August 2014). "Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial" (PDF). The Lancet. Oncology. 15 (9): 975–85. doi:10.1016/S1470-2045(14)70240-2. PMID 24974051.
- ^ a b c d e f
 This article incorporates text from this source, which is in the public domain: "FDA approves darolutamide for non-metastatic castration-resistant prostate cancer". U.S. Food and Drug Administration (FDA) (Press release). 31 July 2019. Archived from the original on 23 November 2019. Retrieved 22 November 2019.
This article incorporates text from this source, which is in the public domain: "FDA approves darolutamide for non-metastatic castration-resistant prostate cancer". U.S. Food and Drug Administration (FDA) (Press release). 31 July 2019. Archived from the original on 23 November 2019. Retrieved 22 November 2019.
- ^ a b c Fizazi K, Albiges L, Loriot Y, Massard C (2015). "ODM-201: a new-generation androgen receptor inhibitor in castration-resistant prostate cancer". Expert Review of Anticancer Therapy. 15 (9): 1007–17. doi:10.1586/14737140.2015.1081566. PMC 4673554. PMID 26313416.
- ^ a b c d e f g h i j k l m Moilanen AM, Riikonen R, Oksala R, Ravanti L, Aho E, Wohlfahrt G, et al. (July 2015). "Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies". Scientific Reports. 5: 12007. Bibcode:2015NatSR...512007M. doi:10.1038/srep12007. PMC 4490394. PMID 26137992.
- ^ a b Shore ND (June 2017). "Darolutamide (ODM-201) for the treatment of prostate cancer". Expert Opinion on Pharmacotherapy. 18 (9): 945–952. doi:10.1080/14656566.2017.1329820. PMID 28490267. S2CID 20624649.
- ^ a b Crawford ED, Schellhammer PF, McLeod DG, Moul JW, Higano CS, Shore N, et al. (November 2018). "Androgen Receptor Targeted Treatments of Prostate Cancer: 35 Years of Progress with Antiandrogens". The Journal of Urology. 200 (5): 956–966. doi:10.1016/j.juro.2018.04.083. PMID 29730201. S2CID 19162538.
- ^ a b "Androgen receptor modulating compounds".
- ^ a b
 This article incorporates text from this source, which is in the public domain: "Drug Trials Snapshots: Nubeqa". U.S. Food and Drug Administration (FDA). 9 August 2019. Archived from the original on 23 November 2019. Retrieved 22 November 2019.
This article incorporates text from this source, which is in the public domain: "Drug Trials Snapshots: Nubeqa". U.S. Food and Drug Administration (FDA). 9 August 2019. Archived from the original on 23 November 2019. Retrieved 22 November 2019.
- ^ "AusPAR: Darolutamide". Therapeutic Goods Administration (TGA). 3 August 2020. Retrieved 22 September 2020.
- ^ Hird AE, Magee DE, Bhindi B, Ye XY, Chandrasekar T, Goldberg H, et al. (October 2020). "A Systematic Review and Network Meta-analysis of Novel Androgen Receptor Inhibitors in Non-metastatic Castration-resistant Prostate Cancer". Clin Genitourin Cancer. 18 (5): 343–350. doi:10.1016/j.clgc.2020.02.005. PMID 32278840. S2CID 215748376.
- ^ Mori K, Mostafaei H, Pradere B, Motlagh RS, Quhal F, Laukhtina E, et al. (November 2020). "Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis". Int J Clin Oncol. 25 (11): 1892–1900. doi:10.1007/s10147-020-01777-9. PMC 7572325. PMID 32924096.
- ^ Halabi S, Jiang S, Terasawa E, Garcia-Horton V, Ayyagari R, Waldeck AR, et al. (August 2021). "Indirect Comparison of Darolutamide versus Apalutamide and Enzalutamide for Nonmetastatic Castration-Resistant Prostate Cancer". The Journal of Urology. 206 (2): 298–307. doi:10.1097/JU.0000000000001767. PMID 33818140.
- ^ a b Wenzel M, Nocera L, Collà Ruvolo C, Würnschimmel C, Tian Z, Shariat SF, et al. (May 2021). "Overall survival and adverse events after treatment with darolutamide vs. apalutamide vs. enzalutamide for high-risk non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis". Prostate Cancer and Prostatic Diseases. 25 (2): 139–148. doi:10.1038/s41391-021-00395-4. PMC 9184262. PMID 34054128.
- ^ Agarwal N, Di Lorenzo G, Sonpavde G, Bellmunt J (September 2014). "New agents for prostate cancer". Annals of Oncology. 25 (9): 1700–9. doi:10.1093/annonc/mdu038. PMID 24658665.
- ^ Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU International. 91 (5): 455–61. doi:10.1046/j.1464-410x.2003.04026.x. PMID 12603397. S2CID 8639102.
- ^ a b c Fizazi K, Smith MR, Tombal B (October 2018). "Clinical Development of Darolutamide: A Novel Androgen Receptor Antagonist for the Treatment of Prostate Cancer". Clinical Genitourinary Cancer. 16 (5): 332–340. doi:10.1016/j.clgc.2018.07.017. PMID 30197098.
- ^ a b c d Tombal BF, Gomez-Veiga F, Gomez-Ferrer A, López-Campos F, Ost P, Roumeguere TA, et al. (January 2024). "A Phase 2 Randomized Open-label Study of Oral Darolutamide Monotherapy Versus Androgen Deprivation Therapy in Men with Hormone-sensitive Prostate Cancer (EORTC-GUCG 1532)". Eur Urol Oncol. 7 (5): 1051–1060. doi:10.1016/j.euo.2024.01.009. PMID 38272747.
In the darolutamide arm, the median (range) value of testosterone was 413 (209.0–1183.0) ng/dl at baseline and increased by 43.3% (5.7–144.0%) to a median (range) of 595.0 (260.0–1500.0) ng/dl at week 24. In the GnRH arm, the median (range) value of testosterone was 333.0 (210.9–844.0) ng/dl at baseline and decreased by –96.0% (–99.6% to –80.8%) to a median (range) of 12.9 (1.2–52.8) ng/dl at week 24. Figure 3 shows the absolute change in testosterone values from baseline over time for the two arms. [...] The median testosterone level increased by 43.4% at 24 wk. In comparison, testosterone increased by 114.3% at week 25 with enzalutamide monotherapy in the phase II trial of enzalutamide. This difference and its potential consequences will need to be studied further.
- ^ Strauss III JT, Barbieri RL (28 August 2013). Yen & Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 688–. ISBN 978-1-4557-5972-9.
- ^ Tyrrell CJ, Denis L, Newling D, Soloway M, Channer K, Cockshott ID (1998). "Casodex 10-200 mg daily, used as monotherapy for the treatment of patients with advanced prostate cancer. An overview of the efficacy, tolerability and pharmacokinetics from three phase II dose-ranging studies. Casodex Study Group". European Urology. 33 (1): 39–53. doi:10.1159/000019526. PMID 9471040.
- ^ Marcus R, Feldman D, Nelson D, Rosen CJ (8 November 2007). Osteoporosis. Academic Press. pp. 1354–. ISBN 978-0-08-055347-4. Archived from the original on 11 June 2016.
- ^ Mahler C, Verhelst J, Denis L (May 1998). "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clinical Pharmacokinetics. 34 (5): 405–417. doi:10.2165/00003088-199834050-00005. PMID 9592622. S2CID 25200595.
- ^ Gao X, Smith MR, Scher HI, Verholen F, Adorjan P, Dissanayake M, et al. (1 June 2023). "A phase 2, randomized, open-label study comparing the effects of darolutamide versus enzalutamide monotherapy on serum testosterone levels in patients with hormone-naive prostate cancer: ARAMON study". Journal of Clinical Oncology. 41 (16_suppl): TPS5111. doi:10.1200/JCO.2023.41.16_suppl.TPS5111. ISSN 0732-183X.
- ^ Laccetti AL, Smith MR, Scher HI, Verholen F, Adorjan P, Dissanayake M, et al. (1 February 2024). "ARAMON: A phase 2, randomized, open-label study comparing darolutamide (DARO) vs enzalutamide (ENZA) monotherapy on serum testosterone levels in patients (pts) with castration-sensitive prostate cancer (CSPC) after biochemical recurrence (BCR)". Journal of Clinical Oncology. 42 (4_suppl): TPS243. doi:10.1200/JCO.2024.42.4_suppl.TPS243. ISSN 0732-183X.
- ^ Clinical trial number NCT05526248 for "A Study Called ARAMON to Learn to What Extent Does Study Treatment With Darolutamide Affects Testosterone Levels in Men With Prostate Cancer That Had Not Been Treated With Hormonal Therapy Compared to Treatment With Enzalutamide (ARAMON)" at ClinicalTrials.gov
- ^ Sternberg CN, Petrylak DP, Madan RA, Parker C (2014). "Progress in the treatment of advanced prostate cancer". American Society of Clinical Oncology Educational Book. American Society of Clinical Oncology. Annual Meeting (34): 117–31. doi:10.14694/EdBook_AM.2014.34.117. PMID 24857068.
- ^ Zurth C, Graudenz K, Denner K, Korjamo T, Fricke R, Wilkinson G, et al. (2019). "Drug-drug interaction (DDI) of darolutamide with cytochrome P450 (CYP) and P-glycoprotein (P-gp) substrates: Results from clinical and in vitro studies". Journal of Clinical Oncology. 37 (7_suppl): 297. doi:10.1200/JCO.2019.37.7_suppl.297. ISSN 0732-183X. S2CID 87513637.
- ^ Palmieri VE, Roviello G, D'Angelo A, Casadei C, De Giorgi U, Giorgione R (May 2021). "Darolutamide in hormone-sensitive and castration-resistant prostate cancer". Expert Rev Clin Pharmacol. 14 (5): 535–544. doi:10.1080/17512433.2021.1901580. PMID 33685318. S2CID 232159762.
- ^ Zurth C, Koskinen M, Fricke R, Prien O, Korjamo T, Graudenz K, et al. (December 2019). "Drug-Drug Interaction Potential of Darolutamide: In Vitro and Clinical Studies". Eur J Drug Metab Pharmacokinet. 44 (6): 747–759. doi:10.1007/s13318-019-00577-5. PMC 6828636. PMID 31571146.
- ^ Shore N, Zurth C, Fricke R, Gieschen H, Graudenz K, Koskinen M, et al. (October 2019). "Evaluation of Clinically Relevant Drug-Drug Interactions and Population Pharmacokinetics of Darolutamide in Patients with Nonmetastatic Castration-Resistant Prostate Cancer: Results of Pre-Specified and Post Hoc Analyses of the Phase III ARAMIS Trial". Target Oncol. 14 (5): 527–539. doi:10.1007/s11523-019-00674-0. PMC 6797643. PMID 31571095.
- ^ Schinkel AH (April 1999). "P-Glycoprotein, a gatekeeper in the blood-brain barrier". Adv Drug Deliv Rev. 36 (2–3): 179–194. doi:10.1016/s0169-409x(98)00085-4. PMID 10837715.
- ^ Fromm MF (February 2000). "P-glycoprotein: a defense mechanism limiting oral bioavailability and CNS accumulation of drugs". Int J Clin Pharmacol Ther. 38 (2): 69–74. doi:10.5414/cpp38069. PMID 10706193.
- ^ a b c d "Darolutamide - Bayer HealthCare/Orion". Adis Insight. Springer Nature Switzerland AG.
- ^ James L. Gulley (20 December 2011). Prostate Cancer. Demos Medical Publishing. pp. 513–. ISBN 978-1-936287-46-8.
- ^ Leibowitz-Amit R, Joshua AM (December 2012). "Targeting the androgen receptor in the management of castration-resistant prostate cancer: rationale, progress, and future directions". Current Oncology. 19 (Suppl 3): S22-31. doi:10.3747/co.19.1281. PMC 3553559. PMID 23355790.
- ^ a b Clinical trial number NCT02200614 for "Efficacy and Safety Study of Darolutamide (ODM-201) in Men With High-risk Nonmetastatic Castration-resistant Prostate Cancer (ARAMIS)" at ClinicalTrials.gov
- ^ "Darolutamide". ChemIDplus. U.S. National Library of Medicine.
- ^ Chustecka Z (31 January 2020). "Darolutamide (Nubeqa) Gets OK in Europe for Prostate Cancer". MedScape.
- ^ a b Tombal BF, Gillessen S, Loriot Y, Marreaud S, Collette L, Saad F (2018). "Intergroup study EORTC-1532-gucg: A phase 2 randomized open-label study of oral darolutamide (ODM-201) vs. androgen deprivation therapy (ADT) with LHRH agonists or antagonist in men with hormone naive prostate cancer (PCa)". Journal of Clinical Oncology. 36 (6_suppl): TPS406. doi:10.1200/JCO.2018.36.6_suppl.TPS406. ISSN 0732-183X.
- ^ "ODM-201 vs Androgen Deprivation Therapy in Hormone naïve Prostate Cancer". ClinicalTrials.gov. 23 November 2016. Retrieved 16 August 2020.
External links
[edit]- "Darolutamide". Drug Information Portal. U.S. National Library of Medicine.
