Wikipedia:Reference desk/Archives/Science/2008 February 6
| Science desk | ||
|---|---|---|
| < February 5 | << Jan | February | Mar >> | February 7 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
February 6
[edit]How many?
[edit]My friend and I were discussing the rather obscure topic of The Year of the Dolphins when we came upon the rather obscure subject of the dolphin-human ratio. So, to determine that, can anyone tell me how many dolphins there are in the world (all kinds, including Orcas. etc.)? Thank you. 99.226.39.245 (talk) 00:03, 6 February 2008 (UTC)
- Measuring populations is notorious difficult. Measuring marine popoulations is even worse. Consider for example the current controversy of the whale population and fish stocks. Even our estimate of the human population is a very rough guess and we have far better data then we have for any other animal Nil Einne (talk) 11:31, 6 February 2008 (UTC)
- There are almost undoubtedly more humans than all of the whales and dolphins. According to our list of cetaceans there are more than 80 species, and the largest known population is about 3 million for the Pantropical Spotted Dolphin. Most of the species have much smaller population estimates, and the population of many of the species is unknown. If you take the largest numbers possible (90 species, each of 3 million) you come up with the largest conceivable population for all cetaceans of 270 million, or about one quarter of a billion. Since the human population is on the order of 6 billion, the cetaceans if they were the maximum 270 million would be only 1/24th of the human population. (The actual number of cetaceans is probably much smaller than 270 million, of course.)--Eriastrum (talk) 19:06, 6 February 2008 (UTC)
- Thank you very much! 99.226.39.245 (talk) 01:55, 7 February 2008 (UTC)
Extreme biology
[edit]Re steel munching microbes that live in nuclear reactors and shrimp that live in 95ºC, what's the name of the study given to creatures that live in extreme conditions on earth? Thanks in advance, Julia Rossi (talk) 00:24, 6 February 2008 (UTC)
- I'm not sure, but the cretures who live in these conditions are called extremophiles. Hope that's of some help. Zrs 12 (talk) 00:34, 6 February 2008 (UTC)
- That's it exactly – thanks for your help Zrs 12. Julia Rossi (talk) 00:55, 6 February 2008 (UTC)
- You're welcome. Zrs 12 (talk) 01:00, 6 February 2008 (UTC)
- That's it exactly – thanks for your help Zrs 12. Julia Rossi (talk) 00:55, 6 February 2008 (UTC)
what are these yellow flowers called?
[edit]
I have lived in the San Francisco Bay Area my whole life and these yellow flowers sprout out everywhere, but no one i know knows what they are called, and neither do i, but we all know about them. does anyone have any idea? they are all over the place.Boomgaylove (talk) 05:35, 6 February 2008 (UTC)
- I believe what you have there are Oxalis pes-caprae, often known as Bermuda Sorrel (see article for more info). In case you wondered, I found it using this personal website. Sadly, I am not in fact an expert in the field of California wildflowers :) --Bmk (talk) 07:08, 6 February 2008 (UTC)
- Although the California poppy sure is beautiful to see when I'm out on the west coast. --Bmk (talk) 07:13, 6 February 2008 (UTC)
- thank you very much, thats exactly what i wanted. soursob---I KNEW I HAD HEARD THAT SOMEWHERE! apparantly tehyre not california wildflowers though. =( hmm i actually am kinda fond of em, except that out wildflowers are nicer but less common. —Preceding unsigned comment added by Boomgaylove (talk • contribs) 07:36, 6 February 2008 (UTC)
- Can I give you a friendly warning about these plants, I had some in my garden in southern Spain and they are the most pernicious weed I have encountered. They propagate by seed and worse by tiny bulbules attached to the main bulb so if you try to dig them up these bulbules fall of and form dozens more plants. I had to regrettably turn to chemical warfare to control them. Enjoy them where they are. Richard Avery (talk) 08:05, 6 February 2008 (UTC)
- If you need to get rid of them manually, wait until winter. Digging out oxalis then usually means the bulb is contained. After that it crumbles into bulbules as Richard testifies. Julia Rossi (talk) 08:35, 6 February 2008 (UTC)
- They are definitely a terrible invasive plant in coastal California. You should make every effort to rid your garden of them. I live in central coastal California and believe me, I know!--Eriastrum (talk) 19:11, 6 February 2008 (UTC)
- They're considered a noxious weed in Australia. Pity, really; they look very pretty. Another one is Patterson's curse, which looks absolutely magnificent when it invades a whole 1,000-acre paddock. -- JackofOz (talk) 21:50, 6 February 2008 (UTC)
Thermochemistry
[edit]For homework, I was given the following problem: The carbon dioxide exhaled in the breath of astronauts if often removed from the spacecraft by a reaction with lithium hydroxide: 2LiOH(s) + CO2(g) => Li2CO3(s) + H2O(l) Calculate the work involved (in joules) when 7.20g of LiOH(s) reacts with 4.16L of CO2(g) at 1.00 atm and 25.0 degrees C. Assume ideal behaviour for CO2. Is the work done by the system on the surroundings, or the other way around?
I used ideal gas law to find that there's 0.17 mol CO2 initially, which, through stoichiometry, I determined to be the limiting reactant. But what do I do next? I know that work=-P*deltaV, so would I say that the change in volume = -4.16L, and so that the work = 4.16L*atm? I have an issue with this because the surrounding doesn't cause the CO2 to dissipate. —Preceding unsigned comment added by 76.68.246.134 (talk) 06:37, 6 February 2008 (UTC)
- Yes. 1atm = 105Nm-2 and 1Litre=10-3m3 for the answer in Joules. That would be the work done by the LiOH. What you've calculated is the work done on the air (the surroundings) by the LiOH - that seems to be the same as the work extractable (by a piston perhaps) from the system.. Hope that is right and helps.87.102.74.24 (talk) 20:17, 6 February 2008 (UTC)
Scientific reason for men or women higher%?
[edit]In the world’s population , is the % of men greater or women? And in case of any of the possibilities what is it’s scientific reason except the genetic basis of 1: 1 possibility of XX or XY.Moreover if the % of any of these two is greater and the same fashion prevails for the incomming 20-40 years, will it not lead to unbalanced human population and if so what will be the most dramatic result of this change?--Mike robert (talk) 08:07, 6 February 2008 (UTC)
- The article on sex ratio discusses this in some detail.--Shantavira|feed me 08:32, 6 February 2008 (UTC)
Water freezing point and supercooling
[edit]Hi, I posted an unanswered question in the Supercooling talk page ("Can be supercooled"). Can someone please help? Gil_mo (talk) 09:06, 6 February 2008 (UTC)
- In short, there's no good reason to redefine the freezing/melting point of water based on achievable supercooling effects. All that would accomplish is to move (and vastly enlarge) the area of ambiguity. Explaining that water freezes at 0°C except for sometimes in controlled environments is tricky, yes. Contrast this, though, with saying that water freezes at -42°C except for natural environments where it has this pesky problem of pseudofreezing at 0°C. This similarly holds for superheating, freezing vs melting points, and other such issues. — Lomn 17:43, 6 February 2008 (UTC)
- Melting and freezing point are statistically defined. That is, they refer to the phase transition between most probable macrostates in a thermodynamically random sample. If you control the randomness (by cooling really fast), you can achieve less-likely macrostates, and I believe supercooling is an example of this phenomenon. SamuelRiv (talk) 17:53, 6 February 2008 (UTC)
- I'm more confused than I was to start with, since both replies seem quite ambiguous. What is "most probable"? Isn't the freezing point measured under lab conditions, i.e. by taking pure clear lab water (not pond water), cooling it in a most clean container until it freezes? If the answer is yes, then in that case the water would freeze at minus 42 celcius. Gil_mo (talk) 18:01, 6 February 2008 (UTC)
- In its basic formulation statistical mechanics looks at "most likely states" from the perspective that every microstate is equally probable. That means that any potential barrier to crystalline freezing, as occurs in ice, that would require a seed molecule, is ignored. So you are effectively assuming infinite randomness of initial distribution. This can be formalized mathematically, and should create a precise definition of melting and freezing points. You can likely do the same thing with supercooling if you change the "all microstates are equal" axiom, but that would be a lot less useful from a real-world perspective. SamuelRiv (talk) 18:23, 6 February 2008 (UTC)
- I'll repeat my answer from there: We got taught that although melting point is well defined, because of the above "freezing point" is in fact not well defined, period. therefore, there are such things as melting point microscopes for analysis; nobody makes a "freezing point microscope". Even the article I found on "Freezing point determination" depends on, if you read it, actually measuring the melting point Gzuckier (talk) 19:46, 6 February 2008 (UTC)
- Thank you all. Gil_mo (talk) 07:49, 7 February 2008 (UTC)
- I'll repeat my answer from there: We got taught that although melting point is well defined, because of the above "freezing point" is in fact not well defined, period. therefore, there are such things as melting point microscopes for analysis; nobody makes a "freezing point microscope". Even the article I found on "Freezing point determination" depends on, if you read it, actually measuring the melting point Gzuckier (talk) 19:46, 6 February 2008 (UTC)
- In its basic formulation statistical mechanics looks at "most likely states" from the perspective that every microstate is equally probable. That means that any potential barrier to crystalline freezing, as occurs in ice, that would require a seed molecule, is ignored. So you are effectively assuming infinite randomness of initial distribution. This can be formalized mathematically, and should create a precise definition of melting and freezing points. You can likely do the same thing with supercooling if you change the "all microstates are equal" axiom, but that would be a lot less useful from a real-world perspective. SamuelRiv (talk) 18:23, 6 February 2008 (UTC)
- I'm more confused than I was to start with, since both replies seem quite ambiguous. What is "most probable"? Isn't the freezing point measured under lab conditions, i.e. by taking pure clear lab water (not pond water), cooling it in a most clean container until it freezes? If the answer is yes, then in that case the water would freeze at minus 42 celcius. Gil_mo (talk) 18:01, 6 February 2008 (UTC)
- Melting and freezing point are statistically defined. That is, they refer to the phase transition between most probable macrostates in a thermodynamically random sample. If you control the randomness (by cooling really fast), you can achieve less-likely macrostates, and I believe supercooling is an example of this phenomenon. SamuelRiv (talk) 17:53, 6 February 2008 (UTC)
A Gulf Stream in the Pacific?
[edit]The Gulf Stream takes warm water to the north and helps in making the weather of west Europe and East North America warmer than the same latitude in Asia; I want to ask the following:

- Why isn’t there a similar stream in the Pacific?
- Could such a stream exist (or did exist) in the Pacific? Maybe due to global warming?
- How far would the effect of such a stream extend inland (if it’s the same magnitude as the Gulf stream)? E.g. would the effect of such a stream give Magadan a similar climate to Helsinki?
-DelftUser (talk) 10:37, 6 February 2008 (UTC)
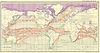
- There is such a current. See the article Ocean current. The north-flowing warm current in the Pacific can't get through the narrow Bering Strait, so its effect is different. It seems to miss the Sea of Okhotsk, too, being deflected by the Kuril Islands. Pity, that. Stupid Kuril Islands. I'm sure Magadan would be a lot more temperate if the current got in there. --Milkbreath (talk) 15:08, 6 February 2008 (UTC)
- So it has to do with geography and not climate! Indeed pity, thanks a lot. --DelftUser (talk) 09:44, 8 February 2008 (UTC)
minimum molecular weight of colloids (IV fluids)
[edit]what is the minimum molecular weight of colloid IV fluids? what is the maximum MW of crystaloids? what is the basis of such classification? if it is based on capillary permiability, what about charged molecules? some books say more than 30000: but, what is the mw of mannitol? i am confused. please help me
dr_sutharshana —Preceding unsigned comment added by 222.165.153.46 (talk) 11:15, 6 February 2008 (UTC)
User:Jcrook1987/Templates/Refdesk Email Removed
physics
[edit]circuit diagram of an opto-electronic lamp system
by tusiime fiona —Preceding unsigned comment added by 82.206.143.13 (talk) 14:11, 6 February 2008 (UTC)
- I'm not sure what you want exactly, but one of the links within Optoelectronics may help. --Bmk (talk) 16:39, 6 February 2008 (UTC)
Hair and nails
[edit]As our Hair and Nails dead?How can it grow?...usman khan —Preceding unsigned comment added by Usmanzia1 (talk • contribs) 14:14, 6 February 2008 (UTC)
- Yes, both hair and nails are 'dead'. They both grow as new material is added at their bases, pushing the hair or nail out. TenOfAllTrades(talk) 14:32, 6 February 2008 (UTC)
- most things which you can cut off your body and not scream are 'dead'. Gzuckier (talk) 19:37, 6 February 2008 (UTC)
- And on the off chance that the question is just badly formatted, there's also this. GeeJo (t)⁄(c) • 20:03, 6 February 2008 (UTC)
K2, the most difficult mountain in the world?
[edit]I've heard many times that the K2 is the "most difficult mountain to climb" in the world, more than mount Everest. Is that true? If it is, why is that? (topographic prominence doesn't seem to be a factor) --Taraborn (talk) 14:24, 6 February 2008 (UTC)
- K2 is much steeper, much windier, much more prone to avalanche, and requires significant technical skill. Everest, by contrast, is technically pretty simple - only the Khumbu Icefall (which paying climbers cover on ladders when roped up) and the Hillary Step (which is roped) pose much of a technical challenge - the rest is just a horrible icey slog (made dangerous largely because of the horrific effects of altitude). K2 has all those same altitude problems, plus is a technically difficult and terribly unpredictable hill. See this for more info. -- Finlay McWalter | Talk 14:37, 6 February 2008 (UTC)
- Excellent response, thanks. --Taraborn (talk) 14:54, 6 February 2008 (UTC)
- Probably the only reason Everest is considered so difficult is because so many Americans used to like the appalachains try to climb it.. don't they realize that Everest is neither the highest point on earth NOR the tallest mountain from its base? :D\=< (talk) 16:59, 6 February 2008 (UTC)
- "Mount Everest is the highest mountain on Earth, as measured by the height of its summit above sea level." Also list of highest mountains -- MacAddct 1984 (talk • contribs) 17:29, 6 February 2008 (UTC)
- Chimborazo_(volcano)#Farthest_point_from_Earth.27s_center ... wtf does the level of the oceans have ANYTHING to do with the highest point? :D\=< (talk) 20:14, 6 February 2008 (UTC)
- Because most humans tend to live their lives in the space between the oceans and the sky, only occasionally going under water, which makes the ocean level a convenient and reasonable reference point when considering humanity's relationship with mountains (or with those parts of mountains that are not under the ocean). -- JackofOz (talk) 21:43, 6 February 2008 (UTC)
- I'll be impressed when someone climbs Olympus Mons -- MacAddct 1984 (talk • contribs) 17:44, 6 February 2008 (UTC)
- Bah. Olympus Mons is a piddling five-degree slope and you'd be under one-third Earth's gravity. I've had walks in the park that are more difficult. I won't be impressed until someone does the climb without supplemental oxygen. TenOfAllTrades(talk) 19:01, 6 February 2008 (UTC)
- :D\=<, the thickness of the air is based on its hight above sea level. The bulge of both sea level and the atmosphere near the center of the Earth are due to the centrifugal effect. The only things about hight that effect the difficulty of a climb are the distance you climb (hight from the base), and the thickness of the atmosphere (hight from sea level). Why would it matter how far you are from the center of the Earth? — Daniel 00:56, 7 February 2008 (UTC)
Homework HELP (DO NOT GIVE ANSWER)
[edit]"How much energy must be added to a 2-kg piece of aluminum with a specific heat of 900 J/(kgoC) to increase its temperature from 10 degrees Celsius to 50 degrees Celsius?"
How do I solve the above problem? At first I thought I used the forumla enthalpy change=final enthalpy-initial enthalpy, but that wasn't right. Please help!!! DO NOT GIVE ME THE ANSWER!!!--AtTheAbyss (talk) 14:48, 6 February 2008 (UTC)
- No, that's not the formula that you need. There's another one that gives an explicit relationship of the variables mass, specific heat, temperature and energy. Just apply that formula, it's a trivial problem. --Taraborn (talk) 14:53, 6 February 2008 (UTC)
- Wait is the equation Joules/(Kg)*(Degrees in Celsius)?--AtTheAbyss (talk) 14:57, 6 February 2008 (UTC)
- Nevermind I figured it out. Thanks anyway though. --AtTheAbyss (talk) 15:01, 6 February 2008 (UTC)
- I meant the one that said Heat=mass*specific heat*change in temperature, if that's of any help now. --Taraborn (talk) 15:02, 6 February 2008 (UTC)
- yeah it does. The answer is 72,000 right?--AtTheAbyss (talk) 15:05, 6 February 2008 (UTC)
- Yes, it is. --Taraborn (talk) 15:06, 6 February 2008 (UTC)
- yeah it does. The answer is 72,000 right?--AtTheAbyss (talk) 15:05, 6 February 2008 (UTC)
- I meant the one that said Heat=mass*specific heat*change in temperature, if that's of any help now. --Taraborn (talk) 15:02, 6 February 2008 (UTC)
- Thanks mate. I owe ya one. --AtTheAbyss (talk) 15:13, 6 February 2008 (UTC)
- You're welcome :) --Taraborn (talk) 19:40, 6 February 2008 (UTC)
Watch out for the problems where there is also one or more phase changes, such as starting with ice and winding up with steam. Edison (talk) 16:10, 6 February 2008 (UTC)
Albumin and 11-year sun cycle
[edit]A friend of mine says that in the 1930s a correlation was found between human Albumin levels and the 11-year solar cycle. I can't find anything about it. Does anyone have any information on that? Bubba73 (talk), 18:20, 6 February 2008 (UTC)
- My friend sent me this
Has this been verified or refuted? Bubba73 (talk), 19:32, 6 February 2008 (UTC)Lowered human immunity may also be a consequence of solar activity, according to Solco W. Tromp, director of the Biometeorological Research Center in the Netherlands. Over 30 years of research, using blood data from 730,000 male donors, led Tromp to the conclusion that the blood sedimentation rate varies with the sunspot cycle. Since this rate parallels the amount of albumin and gamma globulin, resistance to infection may also follow the lead of the sun. (Freitas, Robert A., Jr.; "Sunspots and Disease," Omni, 6:40, May 1984.)
- A link to read. Bubba73 (talk), 19:38, 6 February 2008 (UTC)
ailments due to marriage in close relations????
[edit]what if I marry a girl whose father's maternal grandmother & my grandfather were real brother and sisters,then what health hazards we both can face??? —Preceding unsigned comment added by 117.99.5.120 (talk) 18:54, 6 February 2008 (UTC)
- You are referring to a second-cousin marriage. See cousin couple which specifies the genetic risk for first-cousin marriages. The genetic risk for second-cousin marriage will be less. -- kainaw™ 19:40, 6 February 2008 (UTC)
- Is this not a "first-cousin-once-removed marriage?" —BradV 22:37, 6 February 2008 (UTC)
- Nope. The grandfather and grandmother are siblings. Their children are cousins to each other. The next generation of children are second cousins to each other, the next third cousins, and so on. The "removed" comes in between people not of the same generation relative to a common ancestor. -- JackofOz (talk) 22:44, 6 February 2008 (UTC)
- They're not the same generation. Her great-grandmother was sister to his grandfather. They're second cousind once removed. --Trovatore (talk) 22:48, 6 February 2008 (UTC)
- Oops. You're correct, Trovatore. I misread it as "whose maternal grandmother", not "whose father's maternal grandmother". That makes the questioner and the girl's father second cousins; and the questioner and the girl herself second cousins once removed. -- JackofOz (talk) 23:02, 6 February 2008 (UTC)
- Ok, so they're even further than second cousins. The common ancestors are the OP's great-grandparents and the girl's great-great-grandparents. I believe that makes the consanguinity around 2%. —BradV 23:45, 6 February 2008 (UTC)
- Oops. You're correct, Trovatore. I misread it as "whose maternal grandmother", not "whose father's maternal grandmother". That makes the questioner and the girl's father second cousins; and the questioner and the girl herself second cousins once removed. -- JackofOz (talk) 23:02, 6 February 2008 (UTC)
- They're not the same generation. Her great-grandmother was sister to his grandfather. They're second cousind once removed. --Trovatore (talk) 22:48, 6 February 2008 (UTC)
- Nope. The grandfather and grandmother are siblings. Their children are cousins to each other. The next generation of children are second cousins to each other, the next third cousins, and so on. The "removed" comes in between people not of the same generation relative to a common ancestor. -- JackofOz (talk) 22:44, 6 February 2008 (UTC)
- You yourself do not face any health hazards, it would be your kids. If your shared relative had a rare recessive allele to a nasty disease there is a 50% chance that it will be passed on each generation. There is always a chance that you both carry the disease allele (for you ~13% chance for her ~7% chance). Your kids would have a small chance of inheriting two of the disease alleles (one from each of you). Knowing that, you can calculate the probability of your kids inheriting two of the hypothetical alleles. David D. (Talk) 19:45, 6 February 2008 (UTC)
- Why would they have different chances of having the allele? :D\=< (talk) 20:10, 6 February 2008 (UTC)
- Different number of generation. Read the question carefully, it's only his grandparent but her great grand parent who were siblings. David D. (Talk) 21:39, 6 February 2008 (UTC)
- Why would they have different chances of having the allele? :D\=< (talk) 20:10, 6 February 2008 (UTC)
(Edit conflict) To froth: here's why:
Common ancestor -> the girl's great grandmother -> the girl's grandmother -> the girl's father -> the girl. Common ancestor -> the boy's grandfather -> the boy's parent -> the boy.
- The probability of the girl inheriting a given nasty gene from the common ancestor is 1/24 = 1/16 (~7%)
- The probability of the boy inheriting a given nasty gene from the common ancestor is 1/23 = 1/8 (~13%)
To the original questioner: We should not be giving genetic counseling here on the refdesk. If you have any specific worries about marrying the girl, please consult your doctor. --NorwegianBlue talk 21:54, 6 February 2008 (UTC)
Why are Britney and Jamie Lynn Spears both pretty, while their parents are both extremely ugly?
[edit]How is this possible? 64.236.121.129 (talk) 21:31, 6 February 2008 (UTC)
- Hybrid vigor could account for it, also known as heterosis. David D. (Talk) 21:41, 6 February 2008 (UTC)
- Not to mention the fact that "prettiness" is only partly determined by genes. - Nunh-huh 21:57, 6 February 2008 (UTC)
- 64.126... Your comment about the celebrities' parents are obviously subjective as there is no standard of "prettiness" or "ugliness". Which are you by the way, pretty or ugly? Check with mirrior mirror on the wall... ;-) --hydnjo talk 22:02, 6 February 2008 (UTC)
- Possibly dominant and recessive genes? (is that the proper terminology?) Ilikefood (talk) 22:18, 6 February 2008 (UTC)
- It's the right terminology but it doesn't apply here. Something as complicated as "beauty" is not controlled by a few Mendelian characters. --24.147.69.31 (talk) 23:02, 6 February 2008 (UTC)
- Possibly dominant and recessive genes? (is that the proper terminology?) Ilikefood (talk) 22:18, 6 February 2008 (UTC)
- 64.126... Your comment about the celebrities' parents are obviously subjective as there is no standard of "prettiness" or "ugliness". Which are you by the way, pretty or ugly? Check with mirrior mirror on the wall... ;-) --hydnjo talk 22:02, 6 February 2008 (UTC)
- Not to mention the fact that "prettiness" is only partly determined by genes. - Nunh-huh 21:57, 6 February 2008 (UTC)
- Plastic surgery and airbrushing could account for it. --Carnildo (talk) 22:56, 6 February 2008 (UTC)
- Right. Remember that the "pretty" ones here have been largely made to be so (it helps that they are young). Britney in particular makes this level of gauze fairly obvious these days—compare how she looks on the cover of tabloids to the way she looks when she's being presented by her label. Personally I think both Britney and her sister look very plain—take away the makeup, the money, the lights and the fame, and they're no different from many of the girls who went to my high school, unremarkable, couldn't pick them out of a blond crowd. But of course tastes differ. --24.147.69.31 (talk) 23:02, 6 February 2008 (UTC)
- Apparently Britney's and Jamie Lynn's Dads didn't think their girlfriends were too ugly to make them their wives. Your question is based on the premise that these ladies "are extremely ugly"; that's an opinion, not a statement of fact, and the real question is "why do some people think they're ugly but others don't". -- JackofOz (talk) 00:05, 7 February 2008 (UTC)
- "Too ugly"? I would just like to point out that it's possible for one person to think another is ugly and still want to marry them. As to any specific people, I absolutely have no comment! --Anon, 00:26 UTC, February 7, 2008.
- Right, right. All very correct and good. Eye of the beholder and all that. But when the argle-bargle dies down, we all wanted to have those medium-hot high-school honeys at the time, it's undeniable that the nubile BS had a certain trailer-trash, jailbait schwing factor, and her folks are enough to make a freight train take a dirt road. I always thought it was just a lucky roll in the genetic crapshoot. --Milkbreath (talk) 22:33, 7 February 2008 (UTC)
- By some strange law of probability that crapshoot seems repeatable when you compare the early Courtney Love and her dad. ; ) Julia Rossi (talk) 10:14, 8 February 2008 (UTC)
- good eye makeup. look at any beauty star in a paparazzi shot without makeup, you wouldn't give them a second look. Gzuckier (talk) 15:52, 8 February 2008 (UTC)
Of course, you have to take into account that Britney's mum is much older than Britney. And the way Britney is going, in about 5 years her mum is the one who is going to look better. As for the general consideration, my answer to this, as to so many other things, is that it is "regression to the mean". Britney was VERY good looking to begin with, and talented, that is why she became famous. Now when we compare her with others, we have to remember that she was chosen from amongst millions to begin with, so that of course she looks better. She is not a good example to use when looking at how kids look better or worse then their parents. This is the answer to a lot of questions of this kind. For example: why are sequels to films so rarely as good as the originals. Well, look at it this way. For a film to be in the running to get a sequel it must have been a big success to start with. So maybe such films are already in the top 10 % as far as quality is concerned. Now, even if the quality of films was an entirely an arbitrary and random process, then we should find that films of exceptional quality would be followed by sequels of inferior quality. Another example. Why do very tall people marry people who are shorter? And why do geniuses marry people who are less intelligent? It’s commonsense really, isn’t it?
There is no reason to posit causative agencies when the effect may be nothing more than a statistical artefact. Regression to the mean. Once you know how it works, you will see it operating everywhere. Myles325a (talk) 06:41, 11 February 2008 (UTC)
One reason why you think they are pretty is because they are famous. It is the same reason why people like name brand products over generics. Another reason is you have never seen either one first thing in the morning. When you do see them they are on TV with a professional make-up job.cris
Determination of chloride in sea water
[edit]Hi. I'm currently in an AP Chemistry course at my high school. We received a lab that we ourselves have to write the procedure for. The purpose is to determine the percent of chloride in sea water samples. The materials we are given are: 1 ml pipette, test tubes, graduated cylinders, beakers, analytical balance, NaCl solid, silver nitrate solution (unknown concentration), distilled water, potassium chromate solution (unknown concentration, and sea water samples with unknown concentrations. I know that this lab will b a titration- using silver nitrate as the titrant, and potassium chromate as the indicator. My question is- without knowing the molarity of the titrant how is it possible to conduct this experiment and yield resuluts that are accurate?Is there a way I can determine the molarity of the potassium and silver solutions? —Preceding unsigned comment added by 76.214.199.203 (talk) 21:50, 6 February 2008 (UTC)
- Why don't you use the NaCl solid and distilled water to make a known concentration of salt water? Then, use that to determine the concentration of the sodium nitrate. Finally, use that knowledge to determine the concentration of the unknown sea water. (EhJJ) 00:40, 7 February 2008 (UTC)
- Silver Chloride is pretty much insoluble in water. Add excess AgNO3, you get solid AgCl. Wiegh it. n=m/M and you know the moles of Chloride! —Preceding unsigned comment added by Shniken1 (talk • contribs) 02:11, 7 February 2008 (UTC)
storms
[edit]How can a series of synoptic weather maps be used to predict the future location of a low pressure area? —Preceding unsigned comment added by 24.90.241.2 (talk) 21:54, 6 February 2008 (UTC)
Catalase
[edit]Hi there, I've got a couple of questions of Catalase not answered int the article. Since the reaction it catalyses is primarily between the H202 molecule and the Fe2+ ion, and Catalase has four Fe2+'s, does catalase have four active sites? Also, although Catalase has an optimum of around 7.0 apparently, is there a larger range over which the pH is effectively optimum? Thanks, James —Preceding unsigned comment added by J.Lillington (talk • contribs) 21:58, 6 February 2008 (UTC)
I don't know about this enzyme but generally enzymes do not have an active site per metal centre, so no there are probably not 4 active sites. I would guess there would be 2, this is a 'normal' case where two metals are needed at an active site. One is oxidised the other is reduced (I think this is the 'norm' anyway).Some times enzymes have a metal centre that is not involved in the reaction at all but this is normally Zinc (d10 atom and is redox inactive). As for activity in pH, http://www.ualberta.ca/~edtechpd/documents/activity_probes_experimentenzymeaction_boora_v1.pdf on page 8 there is a small graph of this. I'm sure there are better graphs than this though...Shniken1 (talk) 23:21, 6 February 2008 (UTC)
- Catalase is constructed of four identical subunits, so I think it's a safe bet it has four active sites. As for the pH, the value given in the Catalase article has been changed so many times (without changing the citation!) that I have no idea what it should actually say. I've looked on the google scholar, but found a lot of conflicting information (a lot of studies were also on different catalases). Someguy1221 (talk) 23:19, 6 February 2008 (UTC)
- The BRENDA enzyme database points to a JBC paper [1], which gives a pH optimum of 7.5 for Escherichia coli catalase (note that catalases from other species may have different pH optima). Looking at the pH vs. activity graph (Figure 6, black circles), there's little change in activity between 6 and 7.5. It would probably be most correct to say that catalase has a broad pH optimum, centered around pH 7 or so. -- 128.104.112.12 (talk) 00:00, 12 February 2008 (UTC)
23.5°
[edit]Hello. I know that Earth's ever-changing axial tilt is 23.439281°. However, according to my school textbook, Earth's axial tilt is 23.5°. Assuming that Earth is on a 23.5° tilt, why are the latitude distance between the North Pole and the Arctic Circle, Earth's axial tilt, and the most northerly latitude where the Sun's vertical rays reach on the Summer Solstice all 23.5°? The figures may be approximate. Thanks in advance. --Mayfare (talk) 22:13, 6 February 2008 (UTC)
- Does this maybe have something to do with precession? —BradV 22:40, 6 February 2008 (UTC)
- I don't believe precession is relevant here. Rather, it's the geometry of the Earth's tilt relative to its orbit about the Sun. Assume for illustration that the axial tilt is 0°. In this case, the North Pole is the point at which the Sun never rises above the horizon (assuming a point-source Sun) and the Equator marks the arc where the Sun's rays fall vertically. Right? Now let's add the Arctic Circle and the Tropic of Capricorn, without adjusting the axial tilt. The AC is an "arc" at the Pole, and the ToC an arc directly over top of the Equator. Next, let's bump the axial tilt up to 5°. Now at mid-winter the North Pole is 95° off the angle of the Sun's rays, making the Sun-on-the-horizon limit (90° off the ray angle) at 85° latitude. This becomes the line we define as the Arctic Circle. Similarly, at the summer solstice, the Equator is -5° above the ray angle, making the Sun-directly-overhead limit at 5° latitude -- what we define as the Tropic of Capricorn. This 5° relationship (or the 0° relationship in the simplified example) exists because the axial tilt defines other parameters you're asking about. — Lomn 23:44, 6 February 2008 (UTC)
- On a marginally-related-at-best note, I would caution you to beware of false precision when claiming that you "know that Earth's ever-changing axial tilt is 23.439281°." As you've noted (in the phrase!), it's changing, and 8 significant digits are unlikely to be correct. Measurable nutations cycle in as little as 6 days. — Lomn 23:51, 6 February 2008 (UTC)
Evaporation of sea water
[edit]If there were no rain and if all the rivers on earth were to stop flowing to the oceans for ONE day, how much of sea water would evaporate? How much would the sea level lower? 128.163.80.161 (talk) 22:22, 6 February 2008 (UTC)
- Without rain there would be little or no evaporation, as the atmosphere would quickly reach saturation. The article Water cycle says that a total of 505,000 km3 of water falls as precipitation each year, which would be the same as the total annual evaporation. On an average day then there is a total of 1,383.56 km3 of evaporation. I don't know what percentage of that comes from the ocean, but I imagine it would be in the order of 90%. —BradV 23:03, 6 February 2008 (UTC)
- If we taken the premise of the question as actually possible I would imagine a lot of water would start being lost to space. Water is a VERY good green house gas and the earths temperature would increase significantly and the water molecules may get hot enough to be able to escape the earth.Shniken1 (talk) 23:10, 6 February 2008 (UTC)
- Following on from the first response. If there is 1,383.56 km3 of evaporation, and the water surface of the earth is approximately 361,132,000 km² (from Earth), then the average lowering of sea level will be 1383.56/361132000 = 3.83e-6 km, which is 3.83 millimeters.--Dacium (talk) 23:23, 6 February 2008 (UTC)
Thank you all!128.163.174.150 (talk) 23:20, 7 February 2008 (UTC)
NEWTONS LAW 1 AND 2 QUESTIONS FROM THE PHYSICS MAGAZINE GUY
[edit]Hey,it`s Me The Physics Magazine I`ve got some questions for you. the unbalanced force required to accelerate a 2.0 kg mass at 4.0m/s is a 6.0 n b.2.0 n c 8.0 n d 16 n a force of 10.n applied to a given mass accelerates it at 1.0 m/s.the same force applied to a mass one-half as great would produce an acceleration of 1.0 m.s squared 2.0 m.s squared 0.50 m.s squared 4.0m/s squared a certain net force causes a 10.kg mass to accelerate at 20.m/s squared.the same force will cause a 5.0 kg mass to accelerate at 9.8m/s squared 10.m/s squared 25 m/s squared 40.m/s squared. a 30 kg child exerts a force of 100 n on a 50 kg object.the force the object exerts on the child is 0.0n 100n 980n 1500n if the mass of an object is decreased,it`s inertia decreases increases remains the same if the net force applied in the direction of motion to a certain object on a horizontal fritctionless surface is doubled,the acceleration of the object is halved doubled unchanged quadrupled a car whose mass is 2000 kg is accelerated uniformity from rest to a speed of 15m/s in 10.s on a level highway.the net force accelerating the car is 2000n 3000n 20,000n 30,000n if the sum of all the forces acting on a car is zero,the car must be at rest may be at rest must be moving at a constant speed must be accelerating which of the following is equal to one newton kg.m/s kg.m kg.ms 2 kg/ms 2 if the magnitude of the gravitional force of earth on the moon is f,the magnitude of the gravitional force of the moon on earth is smaller than f larger than f equal to f there is also a series of questions,about the asteriod apophis,which set to hit the earth on april 13,2029. a force of 10.n appiled to a given mass accelerates it at 10 m/s squared.the same force to a mass of one half as great would produce an acceleration of 1.0 m/s squared 2.0 m/s squared 0.50m/s squared 4.0m/s squared a certain net force causes a 10.kg mass to accelerate at 2.0 m/s2. the same force will cause a 5.0 kg mass to accelerate at 9.8 m/s squared 2.0 m/s squared 0.50 m/s squared 4.0 m/s squared a certain net force causes a 10 kg mass to accelerate at 20.m/s squared.the same force willcause a 5.0 kg mass to accelerate at 9.8 m/s squared 10 m/s squared 25 m/s squared 40m/s squared a 30 kg child exerts a force of 100 n on a 50 kg object.the force the object exerts on the child is 0.0n 100n 980n 1500n if the mass of an object is decreased,it`s inertia decreases increases remains the same if the net force applied in the direction of motion to a certain object on a horizontal fricionless surface is doubled,the acceleration of the object is halved doubled unchanged quadrupled a car whose mass is 2000 kg is accelerated uniformity from rest to a speed of 15 m/s in 10 seconds on a level highway.the net force accelerating the car is 2000n 3000n 20,000n 30,000n if the sum of all the forces acting on a car is zero,the car must be at rest may be at rest must be involving at a constant speed must be accelerating which of the following is equal to one newton kg.m/s kg.m kg.m/s squared kg/m.s squared the weight of an apple is closet to 1n 9.8n 19.6n 980n on the surface of a distant planet a 5kg mass weighs 20n.what is the acceleration due to gravity on that planet 0.25m.s squared 1m/s squared 4m/s squared 15 m/s squared the unbalanced force required to accelerate a 2.0 kg mass at 4.0m/s squared is 6.0n 2.0n 8.0n 16n —Preceding unsigned comment added by Yeats30 (talk • contribs) 23:49, 6 February 2008 (UTC)
- Please don't yell at us, Physics Magazine Guy. Maybe try to make your question a bit more readable? And did has anyone established if this magazine is real yet? --Emery (talk) 00:02, 7 February 2008 (UTC)
- Changed post to lowercase - much easier to read. By the way, this looks a lot like homework. —BradV 00:13, 7 February 2008 (UTC)
- Thanks BradV and, how did you do that! --hydnjo talk 03:02, 7 February 2008 (UTC)
- If you're on something unixy, pasting the post into "perl -ne 'print lc'" will lowercase it. --Sean 14:55, 7 February 2008 (UTC)
- What I actually did was less impressive. I copied the text into OpenOffice and used the change case feature. —BradV 21:26, 7 February 2008 (UTC)
- If you're on something unixy, pasting the post into "perl -ne 'print lc'" will lowercase it. --Sean 14:55, 7 February 2008 (UTC)
- Thanks BradV and, how did you do that! --hydnjo talk 03:02, 7 February 2008 (UTC)
- Indeed, it is. It's a direct copy and paste job from here. TenOfAllTrades(talk) 01:05, 7 February 2008 (UTC)
- Thanks TOAT for your vigilance and researching. --hydnjo talk 02:53, 7 February 2008 (UTC)
- (edit conflict) Wow! PMG, shame shame. What physics magazine copies homework? Haha. Anyway, consider formatting the values in LaTeX mathematical notation using the tags <math>...</math>. It would be much easier to read. (By the way, don't cheat! You don't learn anything that way.) Zrs 12 (talk) 01:21, 7 February 2008 (UTC)
- Yay Zrs 12! Thank you for your honest assessment. --hydnjo talk 02:39, 7 February 2008 (UTC)
- To be honest I'm kind of disappointed. Up until now "Physics Magazine Guy" has been pretty careful not to let us know we were doing his homework. —BradV 02:58, 7 February 2008 (UTC)
- For shame indeed. Not even the barest attempt to reformat the copied homework problems - a magnificent lack of effort. I second Zrs 12 - learning how to do the problems yourself will be much more rewarding, not to mention much more efficient!! --Bmk (talk) 07:06, 7 February 2008 (UTC)
- On the other hand, Physics Magazine Guy, I encourage you to come back here with specific questions about problems after you've tried them yourself. Or ask your teacher for extra help. --Bmk (talk) 21:28, 7 February 2008 (UTC)
- I second that, BMK. However, PMG, we are here to help, but not just blatantly give answers. Zrs 12 (talk) 03:11, 8 February 2008 (UTC)
- On the other hand, Physics Magazine Guy, I encourage you to come back here with specific questions about problems after you've tried them yourself. Or ask your teacher for extra help. --Bmk (talk) 21:28, 7 February 2008 (UTC)
- For shame indeed. Not even the barest attempt to reformat the copied homework problems - a magnificent lack of effort. I second Zrs 12 - learning how to do the problems yourself will be much more rewarding, not to mention much more efficient!! --Bmk (talk) 07:06, 7 February 2008 (UTC)
- You're right... I searched last time and had a look at the site and haven't yet found where the previous question on dropping a penny and a Mexican hat came from. I guess what happened is he/she gave up trying to be sneaky after it became clear everyone was ignoring him/her anyway Nil Einne (talk) 06:37, 8 February 2008 (UTC)
