Carbon subsulfide
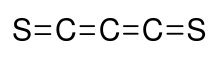
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Propa-1,2-diene-1,3-dithione | |
| Other names
Carbon Subsulfide, Tricarbon Disulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3S2 | |
| Molar mass | 100.15 g·mol−1 |
| Appearance | red liquid |
| Density | 1.319 g cm−3[1] |
| Melting point | −1 °C (30 °F; 272 K) |
| Boiling point | 90 °C (194 °F; 363 K) |
| insoluble | |
| Related compounds | |
Related compounds
|
Carbon suboxide Carbon disulfide Ethenedithione |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Carbon subsulfide is an organic, sulfur-containing chemical compound with the formula C3S2 and structure S=C=C=C=S. This deep red liquid is immiscible with water but soluble in organic solvents. It readily polymerizes at room temperature to form a hard black solid.
Synthesis and structure
[edit]C3S2 was discovered by Béla Lengyel,[2] who assigned it an unsymmetrical structure. Later, infrared and Raman spectroscopy showed that the structure is symmetrical with a D∞h point group symmetry,[3] i.e. S=C=C=C=S. This compound is analogous to carbon suboxide whose structure is O=C=C=C=O.
Lengyel first synthesized this compound by passing carbon disulfide (CS2) vapor through an electric arc with carbon electrodes. This treatment produced a black solution that after filtration and evaporation gave a cherry-red liquid. He determined the molecular mass by cryoscopy. Later preparations of C3S2 include thermolysis of a stream of CS2 in a quartz tube heated to 900 to 1100 °C as well as flash vacuum pyrolysis (FVP) of 1,2-dithiole-3-thiones.[4]
Reactions and occurrence
[edit]Among its few known reactions, C3S2 reacts with bromine to form the cyclic disulfide.[5]
C3S2 polymerizes under applied pressure to give a black semi-conducting solid. A similar pressure-induced polymerization of CS2 also gives a black semiconducting polymer.
In addition, reactions of C3S2 can yield highly condensed sulfur-containing compounds, e.g. the reaction of C3S2 with 2-aminopyridine.
Using microwave spectroscopy, small CnS2 clusters have been detected in interstellar medium.[6] The rotational transitions of these molecular carbon sulfides matched with the corresponding molecules.
References
[edit]- ^ Stock, A.; Praetorius, P. (1912). "Zur Kenntnis des Kohlensubsulfids, C3S2". Chemische Berichte. 45 (3): 3568–3578. doi:10.1002/cber.191204503114.
- ^ von Lengyel, B. (1893). "Ueber ein neues Kohlenstoffsulfid". Berichte der Deutschen Chemischen Gesellschaft. 26 (3): 2960–2968. doi:10.1002/cber.189302603124.
- ^ Smith, W. H.; Leroi, G. E. (1966). "Infrared and Raman Spectra of Carbon Subsulfide". The Journal of Chemical Physics. 45 (5): 1778–1783. Bibcode:1966JChPh..45.1778S. doi:10.1063/1.1727828.
- ^ Pedersen, C. T. (1996). "The formation of 1,2-propadiene-1,3-dithione (carbon subsulfide) from flash vacuum pyrolysis of 1,2-dithiole-3-thiones". Tetrahedron Letters. 37 (27): 4805–4808. doi:10.1016/0040-4039(96)00941-0.
- ^ Stadlbauer, W.; Kappe, T. (1999). "The Chemistry of Carbon Subsulfide". Sulfur Reports. 21 (4): 423–445. doi:10.1080/01961779908047951.
- ^ Cernicharo, J.; Guelin, M.; Hein, H.; Kahane, C. (1987). "Sulfur in IRC + 10216". Astronomy and Astrophysics. 181 (1): L9 – L12. Bibcode:1987A&A...181L...9C. ISSN 0004-6361.
