Wikipedia:Reference desk/Archives/Science/2012 March 1
| Science desk | ||
|---|---|---|
| < February 29 | << Feb | March | Apr >> | March 2 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
March 1
[edit]Number 27
[edit]<copyvio image removed>
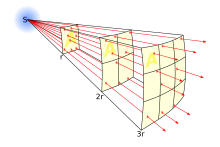
Question 27 reads, "If an X-ray detector measures 1 mW of power from an X-ray binary 2.5 kpc away, how much energy (in mW) would it measure from an equally bright x-ray binary 5 kpc away?"
Can anyone give me the formula or the relationship between power emitted by stars and their distances to Earth? The answer for number 27 is any numbers between .2 to .3 are acceptable. Thanks!Pendragon5 (talk) 00:09, 1 March 2012 (UTC)
| kerfuffle |
|---|
| The following discussion has been closed. Please do not modify it. |
|
- The thing about the inverse square law, if you haven't realized it yet, is that light going through any square in space is projected out onto a square that grows larger the further you go away. And as a result the converse tends to be true - any given speck of a star like our Sun that you see is just as bright no matter where you look at it from! So if you're orbiting Saturn, the Sun is a little speck, but it's just as bright as the little speck you see during a partial eclipse on Earth. Wnt (talk) 05:06, 1 March 2012 (UTC)
- To answer the original question from the OP; no, no one can give you such an equation as there is no relationship between the power emitted by a star and it's distance from earth. 203.27.72.5 (talk) 05:30, 1 March 2012 (UTC)
- That makes no sense to me: Any star at remote distances will effectively be an omnidirectional emittor of electromagnetic radiation (ie sends out radio wave, light, or whatever in all directions). And, as any radio man will tell you, intensity drops with distance per inverse square law, as the further out the recieve antenna/observer eye is, the smaller fraction of the wavefront sphere surface the antenna apperture or eye retina area is, therefore the lower the quantity of radiation /light that is intercepted and detected. So yes, for any star of average star power, there is a relationship between perceived power and distance - the inverse square law. Ratbone58.170.156.97 (talk) 09:07, 1 March 2012 (UTC)
- As a radio man, I can confirm that intensity of a transmitter drops with distance from it, but you are entirely wrong is disputing the claim from 203.7 that "there is no relationship between the power emitted... and distance from earth." The power emitted by a star depends only on its own internal processes and is not changed by moving further away from it. SpinningSpark 13:37, 1 March 2012 (UTC)
- And neither does the power emitted by a radio transmitter change with the distance away the observer is. But the OBSERVED power seen by the distant observer certainly does, which is what I said. I suspect that's what the OP meant: he said "power emitted by stars" but as this taken literally means little sense re the question, he probably meant "the power of the star as observed on Earth", which is not very good prose, but does make sense. You guys get too hung up on various OP's english, as Wickwack said. Ratbone124.178.134.83 (talk) 14:55, 1 March 2012 (UTC)
- As a young aspiring astronomer, the OP should be more careful of language where it counts, and hopefully now realizes where they were either quite sloppy with respect to "emitted" vs. "received" or where they had a serious misunderstanding of the physics behind the question. -- ToE 19:44, 1 March 2012 (UTC)
- That makes no sense to me: Any star at remote distances will effectively be an omnidirectional emittor of electromagnetic radiation (ie sends out radio wave, light, or whatever in all directions). And, as any radio man will tell you, intensity drops with distance per inverse square law, as the further out the recieve antenna/observer eye is, the smaller fraction of the wavefront sphere surface the antenna apperture or eye retina area is, therefore the lower the quantity of radiation /light that is intercepted and detected. So yes, for any star of average star power, there is a relationship between perceived power and distance - the inverse square law. Ratbone58.170.156.97 (talk) 09:07, 1 March 2012 (UTC)
- The core problem here is that the question the OP asked ("is there a relationship between power emitted by stars and their distance to earth"), the question the OP sort-of-intended to ask ("is there a relationship between the observed power of a star and its distance to earth"), and the question that the OP's homework problem asks ("if you take a given star of X power and vary its distance to earth, how will the observed power change") are all very different -- and that many responses above are taking only one at a time in isolation. Taking them in order: there is no useful relationship between emitted power and distance from Earth. We see stars of comparable power outputs both nearby and far away. There is no useful relationship between observed power and distance from Earth. In the absence of other information, a star that appears bright can be objectively-dim-but-close or objectively-bright-but-far. However, if we include all three items -- emitted power, observed power, and distance -- knowledge of any two and handwaving away of external interference lets you calculate the third, per the inverse square law as discussed initially. — Lomn 15:13, 1 March 2012 (UTC)
The question was clear that it was power at a detector - I've typed it in again as text for clarity. In case the OP, or anyone else, hasn't figured out inverse square by now, I'll answer this: it would measure 0.25 mW of power. To see this, consider that a one-inch square detector held out at 2.5 kpc would "project", like the film in a film projector, to a square two inches on a side at 5 kpc. The equation is
Note that 4 pi r2 is the area of a sphere of radius r.
But it's easier to say that "power at distance 1"/"power at distance 2" = ("distance 2"/"distance 1")2 Wnt (talk) 19:26, 1 March 2012 (UTC)
How did ruminant animals evolve?
[edit]
Were there animals at various stages inbetween being fully ruminant and non ruminant? Count Iblis (talk) 02:05, 1 March 2012 (UTC)
- There's an unreferenced assertion that there are pseudo-ruminants (only 3 stomach compartments) in the ruminants article. It doesn't list any examples. 203.27.72.5 (talk) 02:58, 1 March 2012 (UTC)
- I take it the question is how you evolve 4 "stomachs" from less than 4. Conceptually, this is the same as how you evolve the first stomach from an intestine alone. If there was an evolutionary advantage, a pouch might evolve in a spot along the intestines, and food might digest for a while before moving on. Folds at the top and bottom might then evolve, which eventually become sphincters. As many stomachs could evolve in this way as are evolutionarily beneficial. StuRat (talk) 03:18, 1 March 2012 (UTC)
- Indeed the initial pouch probably would have been beneficial, given that one purpose is to allow more time for fermentation of cellulose to occur, which is indeed a slow process. Looking through the literature on ruminants (there are at least 150, it would appear), it is clear that the ruminants in which the food spends more time in the early stomach chambers are more successful at acquiring efficient fermentation of cellulose (there are some ruminants that have no need for complete fermentation as they consume far more nutritive foods than grass and grain). That said, there are a number of animals described as "semi-ruminants", with the Alpaca explicitly stated as having a three-chambered stomach. The other semi-ruminants I found are the colobus, quokka, rabbit and hoatzin. However, the papers I found that use this phrase are not exactly stellar or numerous, and one of those organisms is a freakin' bird! (The rest are at least mammals, but are still no where near the true ruminants in terms of phylogeny.) Clearly these animals arrived at 1 < n < 4 stomachs through convergent rather than divergent evolution. As to the evolution of the stomach chambers themselves, I didn't come across any claims that the semi-ruminants are offshoots of either the ruminants or their common ancestors. I also didn't find anything but unsupported theories on how the stomach evolved to be chambered. Someguy1221 (talk) 05:32, 1 March 2012 (UTC)
- Oh, well, I have to now eat what I just said. I was looking for semiruminants when I should have been looking for pseudoruminants, and here is a review about the pseudruminants: [1]. Within the order Artiodactyla, ruminism evolved independently at least twice, once within tylopoda, where you find the pseudoruminants, and once within Pecora, where you find most of the true ruminants. So, despite being wrong, I was still right - the pseudoruminants are still not evolutionary intermediates to ruminants and non-ruminants, but their own thing. It's interesting though, that they are so closely related to the true ruminants. Someguy1221 (talk) 05:53, 1 March 2012 (UTC)
- Rabbits are not "semi-ruminants" as your source suggests. They are monogastric, hindgut fermenters. 203.27.72.5 (talk) 06:32, 1 March 2012 (UTC)
- I've added an image of mammalian stomachs (from stomach) above. As you see, when you look at just humans, then ruminants, it looks as if stomachs were just plain added (or lost). But looking at the intermediate form, we see that the stomach has a system of graded zones, which actually dates to long before mammals - personally, I would bet it dates back to the Urbilaterian, but alas, I have no fossil data to cite. :( In humans some of the stomach zones are greatly reduced. Mice have almost the same form of stomach, but retain a "forestomach" region which is more specialized for storage of food. In ruminants, I would guess sharp partitions have formed to separate the ruminant stomach into multiple regions. Wnt (talk) 19:42, 1 March 2012 (UTC)
Thanks everyone for their input so far. One thing seems hard to imagine, a ruminant animal will have to chew and swallow the partially digested food again. But how did this happen for the first time? Wouldn't such an animal simply throw up everything that comes out of its stomach? Count Iblis (talk) 01:36, 2 March 2012 (UTC)
- The one animal in 10 million that didn't think it was disgusting had more kids than everyone else :) Someguy1221 (talk) 02:08, 2 March 2012 (UTC)
- Animals don't seem nearly as disgusted by vomit as us. Many birds puke up food for their chicks, and dogs seem happy to lap their own vomit back up again (right before licking their butts and their owner's face), apparently hoping it will stay down the second time. StuRat (talk) 05:28, 2 March 2012 (UTC)
- For that matter, consider pseudorumination. Yes, it's a shock that first time when you look in on the mouse and it is eating a turd. ;) Wnt (talk) 17:46, 2 March 2012 (UTC)
- Dogs will eat turds too, though not usually their own. They seem to like cat turds. Dog holds pinky in the air and says "Ah, yes, a fine aged Siamese, with subtle mouse undertones and just a hint of catnip". StuRat (talk) 21:14, 2 March 2012 (UTC)
- True - I think I remember reading one study on Lake Superior where wolves were eating more scats than anything else. Can't recall the reference though... Wnt (talk) 23:51, 2 March 2012 (UTC)
- My dog thinks Possum poo is a delicacy. HiLo48 (talk) 23:57, 2 March 2012 (UTC)
- I think this article [2] is informative, about proboscis monkeys which have a forestomach (but not a separate forestomach) and regurgitate and rechew food. To humans, which have lost their forestomachs, this behavior is bizarre. While we may be tempted (falsely) to think of monkeys as "more evolved" than cows, this is an instance in which these primates show what is probably a primitive (phylogenetics) trait. Probably, the same behavior existed, at least in small amounts, in the common ancestor of monkeys and ruminants, and the ruminants, finding a need with the grass digestion, turned it into a predominant lifestyle. Wnt (talk) 17:14, 5 March 2012 (UTC)
Is tree-free paper recyclable?
[edit]I had a pizza box that was made of tree-free paper, and according to the website of the company that made it, it was made out of scraps from sugar canes. Is that kind of paper recyclable with regular paper? 184.7.157.90 (talk) 04:07, 1 March 2012 (UTC)
- Sugar cane scraps are known as bagasse, and since it is essentially cellulose, the same as wood pulp I would hazard a guess that yes, it is recyclable. 203.27.72.5 (talk) 04:28, 1 March 2012 (UTC)
- You need to check with whoever is doing your recycling. Some paper recyclers can be a lot more choosy than others with issues over staples, window envelopes, dyes, bindings, and already recycled paper (because the fibres get shorter on each recycling). It is certainly compostable though, and could go in the compost bin instead. SpinningSpark 13:02, 1 March 2012 (UTC)
- My county's recycler isn't choosy at all; they take colored paper, paper with staples and envelope windows, pizza boxes if they don't have excessive food residue (I usually rip off the top half for recycling since the bottom half is almost always cheesy and greasy), wax coated milk cartons and beverage cups, and other stuff that other places won't take. This is according to an insert they included in the monthly water bill when they first started the single-stream recycling vs. the system they had before. 184.7.157.90 (talk) 13:38, 1 March 2012 (UTC)
Asteroid deflection
[edit]I just read a couple of news articles [3][4][5] about 2011 AG5 and posible ways to deflect it and other asteroids that may be at risk of impacting earth. They list a few different ways, but they're all a bit speculative. Wouldn't it be feasible to get some practice in first and start deflecting asteroids around other objects in our region of space? Also, the idea of breaking it up with nukes is pretty much universally panned by scientists because they think it will turn the object into a swarm of smaller objects that will still impact earth and be much harder to stop. Would it really be that much harder to stop with a gravity tractor type solution given that the swarm will still have a center of gravity, and so for some gravitational purposes will still act like a single object? And isn't a swarm of objects more like to burn up in our atmosphere? 203.27.72.5 (talk) 07:09, 1 March 2012 (UTC)
- What is "a gravity tractor"? AndyTheGrump (talk) 07:32, 1 March 2012 (UTC)
- It's described in one of the articles I linked to. It's basically an artificial satellite
in a geostationary orbit around the objectthat is stationary with respect to the object that will slowly exert a gravitational pull to deflect its path around earth. 203.27.72.5 (talk) 07:42, 1 March 2012 (UTC)
- It's described in one of the articles I linked to. It's basically an artificial satellite
- While it would be a good idea to develop the scientific and engineering expertise necessary to deflect asteroids before it becomes a life-or-death crisis, good luck getting the billions (tens of? hundreds of?) needed to fund such an activity. As for breaking up the object with nukes: if it's still gravitationally bound and still needs a gravity tractor or other deflection means, why did you bother with the nukes? They didn't do anything. And while multiple smaller objects should burn up more readily on atmospheric entry, there's a wide gulf between "burns more readily" and "burns sufficiently". Only the latter is important. — Lomn 15:02, 1 March 2012 (UTC)
- "if it's still gravitationally bound and still needs a gravity tractor or other deflection means, why did you bother with the nukes?" No attempt to solve the problem is foolproof and may fail. If the nuke fails you get a massive stream of smaller (but not small enough to be inconsequential) objects, and you realise straight away that it failed and you need to look at something else to deflect the asteroid. If the gravity tractor sits next to the asteroid for 10 years and by then you realise it has failed...you've just lost 10 valuable years.
- Bear in mind, though -- you've already noted that the nuke option has virtually no support among scientists. I agree that no attempt is foolproof, but backup planning efficiently means you don't waste time/effort/money on your last-ditch option before the things ahead of it have been tried. This similarly ignores that gravity tractors and other deflection means are precise and adjustable whereas shooting a nuke at something is a messy one-time action. Further, supposing you end up with a set of smaller-but-not-small-enough objects post-nuke, you're now going to need a gravity tractor or other real solution per new object. — Lomn 21:28, 1 March 2012 (UTC)
- "if it's still gravitationally bound and still needs a gravity tractor or other deflection means, why did you bother with the nukes?" No attempt to solve the problem is foolproof and may fail. If the nuke fails you get a massive stream of smaller (but not small enough to be inconsequential) objects, and you realise straight away that it failed and you need to look at something else to deflect the asteroid. If the gravity tractor sits next to the asteroid for 10 years and by then you realise it has failed...you've just lost 10 valuable years.
I'm interested to know if my reasoning on influencing the path of a swarm of objects is sound. Does the fact that the density has been lowered make any difference? I'm also interested to know if an asteroid (say the dimentions, density, path and velocity of 2011 AG5) can realistically be broken into small enough pieces to burn up. Is there a known limit which meteors must reach to become meteorites? Is the relationship more complicated than just atmospheric penetration is proportional to volume and/or mass and/or velocity? 203.27.72.5 (talk) 20:53, 1 March 2012 (UTC)
- The references given seem to be drawn from popular press interpretations. An astroid has an 'orbit' about a centre of greater mass. The initiation of several nuclear devices in the right place could retard the overall kinetic energy of fragments enough to change their orbit and thus cause them to miss earth. It is a possible option, but it would need to be performed when the asteroid is far enough away for the change in orbit to have a significant effect -(in this case to miss earth). It would also depend on the mass of the asteroid and the the power of the nuclear devices used. Until a better understanding what asteroids are composed of (i.e., loose or closely bound rock) it is difficult to guess at weather a series of small explosions to slow (or speed up) the overall mass down(or up) without braking it up is best, or a few large devices to knock it down (or up) into a different orbit. Loads of little bits entering the Earths atmosphere over a small area could possibly result in much compressive heating at ground level causing wild-fires over a large area. --Aspro (talk) 22:17, 1 March 2012 (UTC)
- We have an article on Asteroid-impact avoidance that you might like. LukeSurl t c 23:54, 1 March 2012 (UTC)
- Thanks for the link. The scientists cited in that article don't seem to be nearly as down on nuclear weapons, and in talking about manipulating the paths of rubble piles with gravitational tractors they essentially answered my other question. 203.27.72.5 (talk) 04:48, 2 March 2012 (UTC)
- We have an article on Asteroid-impact avoidance that you might like. LukeSurl t c 23:54, 1 March 2012 (UTC)
- Another point to note, is that as nuclear devices are these days old hat. For an up and coming scientist however, a government grant to develop new technology in the form of tractors beams etc., will insure that for s/he or it, will have a job for life. Thus, even if the proposal is without realistic merit, it will be trumpeted as the the one and only savour of the human race. --Aspro (talk) 00:15, 3 March 2012 (UTC)
- An up and coming scientist can generally be guaranteed a job for life just by repeating, "blah blah blah climate change blah blah blah," and that doesn't need any realistic merit either. 203.27.72.5 (talk) 07:58, 3 March 2012 (UTC)
- Couldn't you use that to get funding for asteroid deflection then? I mean, an asteroid impact on Earth could cause serious climate change, according to Isaac Asimov's Inferno. – b_jonas 17:38, 3 March 2012 (UTC)
- An up and coming scientist can generally be guaranteed a job for life just by repeating, "blah blah blah climate change blah blah blah," and that doesn't need any realistic merit either. 203.27.72.5 (talk) 07:58, 3 March 2012 (UTC)
- Another point to note, is that as nuclear devices are these days old hat. For an up and coming scientist however, a government grant to develop new technology in the form of tractors beams etc., will insure that for s/he or it, will have a job for life. Thus, even if the proposal is without realistic merit, it will be trumpeted as the the one and only savour of the human race. --Aspro (talk) 00:15, 3 March 2012 (UTC)
- No. Governments like to restrict financing to further 'new' technology in the hope that it will trickle down into general industry. Their modus operandi is not pro-active, instead they react retrospectively; much like fire-fighters. There is perhaps one exception to this, which is in the case of military defence. Here though, is a quantifiable arms race but again its focused on new technology. During the second world war, much was spent on new technology (i.e. air-craft [please don't call them planes] and of course the 'fish' bomb) yet other stuff (Sherman tanks , guns, ships, etc,) were just cheap mass produced utility items. The possibility of total desolation being wrought by astroids, super tsunamis, new diseases (look at how slow they reacted to aids) and so on, are so many-fold that they are ignored – except by Hollywood and the histrionic population in neighboring LA.--Aspro (talk) 19:24, 4 March 2012 (UTC)
Hypersaline sensitivity
[edit]What is the cause for hypersaline sensitivity? When nearly everything tastes too salty. Plasmic Physics (talk) 07:19, 1 March 2012 (UTC)
- Almost everything seems way too salty to me, too. I attribute that to almost everything actually being too salty. That is, just about all restaurant foods and processed foods in grocery stores. I find I have to "cut" salty foods, like a frozen pizza or soup, by adding my own salt-free veggies to both, and more water to the soup. I also drink lots of water each day to dilute all the excess salt I'm forced to consume. The mystery to me is why most people like food that has an unhealthy amount of salt. Apparently it has to do with accessible salt being rare in nature during our evolution, resulting in people trying to get all they can with each meal. StuRat (talk) 08:26, 1 March 2012 (UTC)
- It depends on whether you're talking about a change in someone's sense of taste toward the salty end, or the mere fact that someone has always been sensitive to salt. The former is a fairly uninformative symptom without the subject's medical history, and if this is you we're talking about, you should only trust an answer from a medical professional. For the latter issue, the salt-receptor-bearing taste buds on the human tongue (or the neurons they are connected to in the brain, perhaps) are not hard-wired to produce either a pleasant or an aversive reaction. Sugar receptors are hard wired for pleasantness, and bitter receptors are hard wired for aversion. But the salt receptors are pretty much left for you to figure out on your own, and we inadvertently train ourselves to like or dislike salt in certain quantities through our eating experiences as we live. The concentration of salt you prefer can also be influenced by your own body, and against your will, in reaction to your diet. Someguy1221 (talk) 08:38, 1 March 2012 (UTC)
- It may be that people like salt because they need it. I worked for about 10 years in a comany that had a staff canteen, Good food, subsidised prices, but lots of salt. When I left that company I gradually developed a range of silly seemingly unrelated symptoms such as not needing to pee at all one day, and needing to pee an awfull lot the next. Doctors were a dead loss - ordered lots of tests but turned up nothing useful. Eventually I decided the only thing that's changed is no more lots of salt, so I started puting lots of salt on my food. Symptoms all went away! Next time I saw my doctor for annual checks, told him. He went "Ahh! yes! & mumbled a few medical words. And no, its not due to iodised salt (iodine is added by law in some countries) - the salt I buy is not iodised. Wickwack121.221.208.112 (talk) 08:58, 1 March 2012 (UTC)
I can say that now I prefer less salt because I'm more sensitive towards salt, not the other way around. I wasn't always like that, I used to have the same sensitivity as relatives. The change must have occur over the period of a few weeks. I'm not medically concerned, just curious, so you don't have to worry about me asking for medical advice. Plasmic Physics (talk) 10:20, 1 March 2012 (UTC)
- I recall a radio interview with a "taste engineer" working for Campbell's Soup Company. His job entailed regularly eating his employer's soup as incremental changes were made to the recipe, and he stated that one of his most difficult times came when he was transferred from working on normal soup to working on low-sodium, healthy (re: disgusting, seriously, I've tried it) soup. He said it tasted awful for a few weeks, but thereafter he couldn't recall a difference. Normal soup now tastes overly salty to him. Someguy1221 (talk) 10:25, 1 March 2012 (UTC)
Missing percent daily value on Food Nutritional information
[edit]In North America the nutritional information box on food packages detail the both the amount as well as the percentage of daily recommended intake. But I noticed that the percentage part is missing for cholesterol, sugars, and protein. Why is that?
This site [6] claims that "...percent DV is missing from the protein label because protein insufficiency is not generally thought to be a problem.", which seems to contradict our article on protein. Anonymous.translator (talk) 07:36, 1 March 2012 (UTC)
- Because protein quality (amino acid composition) is more important than protein quanitity in the North American diet. The human body can produce it's own cholesterol. And sugar consumption is rarely a problem per se, and would be tied to daily caloric requirement, which varies widely from individual to individual. Dominus Vobisdu (talk) 08:03, 1 March 2012 (UTC)
- I don't believe there is a minimum requirement for sugars or cholesterol. In the case of sugars (simple carbs), you can just eat starches (complex carbs) instead, and your body will convert those into sugars as needed. In the case of cholesterol, there is good cholesterol, which should have a minimum RDL, and bad cholesterol, which you probably don't need at all. Since the current label just lists total cholesterol, it's quite useless, as would be a minimum RDL for total cholesterol.
- Protein does have a minimum amount you need, and I believe the FDA formerly set that at 55 grams per day and calculated a percentage of RDA accordingly. However, they were accused by vegans and the like of setting the RDA higher than it should be to support meat producers. Rather than let the science set the proper RDA, they just opted to not list one at all. This explains the US labels, but you said "North America". If Canada and Mexico do similar things, I imagine they just copied what the US does. StuRat (talk) 08:16, 1 March 2012 (UTC)
- StuRat, do you have a source for your vegan story? It does sound like something PETA would do but my google-fu found nothing. I'm not sure whether it's politics, the protein quality factor Dominus mentioned, or both. I'm actually from Canada and our label are almost identical to the US ones. In fact I'm kinda surprised that the US labels have mostly metric units on them. Anonymous.translator (talk) 08:42, 1 March 2012 (UTC)
- Couldn't find a source on that, but here's a source putting the FDA RDA at 55 grams of protein: [7]. I believe those nutrition labels came in when the US gov was pushing the metric system, so they chose grams. Since people really didn't have a tradition of measuring fats, protein, carbs, etc., in other units, the metric system caught on, unlike, say, on speed limit signs. StuRat (talk) 05:23, 2 March 2012 (UTC)
- That clears it up, thanks. Since for cholesterol and sugar it's usually a case of over-consumption rather than deficiency, why not have a percent daily maximum recommended level instead? Anonymous.translator (talk) 08:31, 1 March 2012 (UTC)
- Yes, or perhaps a range for each, as some nutrients have both a minimum and maximum, like iron. The FDA seems very slow to update their requirements. It took forever for them to require listing of trans fats, so don't hold your breath for them to list good and bad cholesterol separately any time soon. They still seem mainly in the mode of "ensuring that everyone gets enough good nutrients", while the main problem for Americans appears to be getting too much of the harmful nutrients, like bad cholesterol, trans fats, sugar, and sodium, which cause heart disease, stroke, and diabetes and may contribute to cancer. StuRat (talk) 08:37, 1 March 2012 (UTC)
Electromagnetic railgun firing projectiles at 9,000km/h!
[edit]The US Navy had just conducted a test firing of its BAE Systems electromagnetic railgun (video). After having read articles about the weapon, I'm wondering where the fire came from if there was no chemical explosions in used, and how much G-force, heat and air pressure is involved here. --Sp33dyphil ©hatontributions 09:34, 1 March 2012 (UTC)
- The first comment seems relevant:
Several effects are working here to create that explosion.
First, as stated in a comment, is ohmic heating, created by the current through the projectile.
Second, the speed that the projectile is coming out of the barrel is high enough that the air around the projectile basically combusts, much like the space shuttle re-entering the atmosphere.
Third, there are layers of metal that are vaporizing off the projectile from friction.
The speed record for this thing was slightly over Mach 7 (2520m/s)
- StuRat (talk) 09:41, 1 March 2012 (UTC)
- Some sources say the speed is ~5000m/s, so I don't think 2520m/s is approximate to Mach 7. --Sp33dyphil ©hatontributions 09:47, 1 March 2012 (UTC)
- As far as I'm aware, 5000 m/s is the speed it's supposed to fire at, rather than the speed it ever actually has. Someguy1221 (talk) 10:35, 1 March 2012 (UTC)
- Some sources say the speed is ~5000m/s, so I don't think 2520m/s is approximate to Mach 7. --Sp33dyphil ©hatontributions 09:47, 1 March 2012 (UTC)
"The flames that appear in the video of the test firing come from a combination of electricity arcing across the launcher, shavings of aluminum reacting with the air, and the bullet's hypersonic flight, said Tom Boucher, test director for the railgun at the Naval Surface Warfare Center Dahlgren Division in Virginia." [8]. Apparently the Navy wants to eventually be able to launch 18kg projectiles at 2500m/s. That gives a kinetic energy of around 57MJ or 1.4% of a ton of TNT. 203.27.72.5 (talk) 22:24, 1 March 2012 (UTC)
- You cannot calculate g-force without knowing acceleration, and you cannot calculate acceleration without either the barrel length (distance over which acceleration occurs) or the time it accelerates for. The dimentions of the railgun are not mentioned in any of the articles I read and are likely classified, so I'm going to assume it's 20m. If we're going to the top speed of 9010km/hr then that's ~2500m/s. It takes 0.016 seconds to reach that speed over 20m. The acceleration is therefore 156,250m/s/s which is 15,944 times greater than 9.8m/s/s (i.e. 1 g-force). So if you rode that projectile (and we negate your mass) you would experience 15,944 g-forces.
- The force exerted is 2.8 meganewtons and if we assume that the projectile is 30cm in diameter (~12inch caliber) then the surface area of the flat nose (""The rounds we are firing currently are non-aerodynamic slugs," Ellis said of the testing."[9]) would be 0.07 square meters. That gives a pressure of 40 megapascals or 400 atmospheres. Even for an aerodynamic projectile this would still be the peak pressure at the tip which can be approximated as flat. This applies to air that is on the nose of the projectile as it starts accelerating. Air that is "hit" by the projectile while in the air will have a much greater peak pressure that is dependent upon the impulse time.
- Heat cannot be easily calculated as I have no idea where the aluminum is even coming from let alone how much there is. Also no idea on how much heat is released by those electrical arcs or exactly how much kinetic energy will be lost due to air friction. 203.27.72.5 (talk) 23:15, 1 March 2012 (UTC)
In reference to the scene where a character is killed by a chemical trap, is there such a substance in existance, either liquid or solution, which can destroy biomass in a matter of seconds as seen in this scene. Once triggered, the trap consists of a sprinkler that vigorously sprays an unknown powerfully oxidising liquid onto the head area for several seconds. The liquid proceeds to destroy the muscle tissue and underlying bone structure several inches deep, lasting for about 30 seconds before the liquid is exhausted. I'm sure that if you want to you can find the particular scene on you tube. Thought about something like trifluoro–λ3–chlorane, but I know nothing of how it behaves in a scenario like this. Plasmic Physics (talk) 10:36, 1 March 2012 (UTC)
- I remember that scene. You see stuff like this, and what always seems so ridiculous to me is that it only takes a relatively small amount of "mystery acid" to dissolve right through someone's body. Certain strong acids are certainly capable of completely dissolving a human (although it takes quite a bit longer than 30 seconds), but the acid is neutralized in the reactions that cause the body to dissolve. Any normal acid sprayed on a human face in the quantity seen in the movie would neutralize itself before doing the damage that's shown. The victim may be blinded and scared for life, but the whole front of his head wouldn't just dissolve. There has been the occasional criminal in the last hundred years who dissolves a victim in acid, but you're talking tens of gallons there. Someguy1221 (talk) 10:42, 1 March 2012 (UTC)
What about those types of chemical reactions where a reactions is in a non-equilibrium, oscillating state - where the reaction intermittently favours the reactants and then the products, until the reaction runs out of energy. This would allow the bulk of the biomass to be atomised, and only a small portion to actually neutralise the acid. How fast can the super acids react in any case, e.g. how long long would it take for a one gram sphere of lead to fully dissolve into one mole of acid at STP? Plasmic Physics (talk) 11:05, 1 March 2012 (UTC)
- I haven't seen the scene - provided it leaves behind an equal mass of waste product, it could be some sort of catalyst (not a simple acid, which indeed would neutralize) - probably advanced nanotech e.g. grey goo. The actual possibility of such things is purely hypothetical - there is, thank goodness, nothing like that now. However, if the apparent volume of the people is greatly reduced, you would need something even more exotic - I'm thinking strangelets, primordial black holes, and even those would not reduce the mass. For that, maybe some kind of ultra-high-nanotech fusion reactor that beams out the mass as a massive beam of gammas? Way beyond guesswork. Wnt (talk) 17:06, 1 March 2012 (UTC)
- While I doubt any real-world acid would kill someone that quickly or dissolve that much tissue for its volume, there are acids that can be lethal after very small exposures. In one case ~100mL Hydrofluoric acid exposure to the thighs caused death in an adult male despite immediate rinsing with massive dilution (he jumped in a swimming pool)[10]. 203.27.72.5 (talk) 21:15, 1 March 2012 (UTC)
- And Prussic acid is deadlier than that... Wnt (talk) 00:41, 2 March 2012 (UTC)
- While I doubt any real-world acid would kill someone that quickly or dissolve that much tissue for its volume, there are acids that can be lethal after very small exposures. In one case ~100mL Hydrofluoric acid exposure to the thighs caused death in an adult male despite immediate rinsing with massive dilution (he jumped in a swimming pool)[10]. 203.27.72.5 (talk) 21:15, 1 March 2012 (UTC)
- YouTube clip: http://www.youtube.com/watch?v=JSIvMMZmkSY. PrimeHunter (talk) 22:09, 1 March 2012 (UTC)
Coincidently, I'm also working on a concept similiar to grey goo, I don't know what to call it yet. It is a substance which has liquid properties, but is dry at the same time - it can flow, but it has a low wettability on most surfaces. It is a smart substance, programmable by using reversable photoisomerisation to manipulate the active site to accomodate different targets. It is supposed to be programmed to leach a highly specific target substance from a sample, essentially a perfect recycling nanotechnology, that can be used to recover all the iron from a car wreck, but leave all the lead behind, or recover all the gold from circuit boards, but leave all the copper behind. Maybe I can come up with some kind of acronym. Plasmic Physics (talk) 22:19, 1 March 2012 (UTC)
- Yikes! I worked with that stuff in a dream I had back in the 90s. It was based on protein-like structures with non-natural amino acids, and my part in the project was to work out multiple stable cross-links (like cysteine) so that we could plan links between different molecules/parts of molecules with confidence. Everyone had some kind of HUD and could watch what everyone else was doing. And when the stuff managed to eat little holes through a glass vial a colleague was holding, it was definitely not long until he was transformed into the most gruesome spectacle, moving forward in a sort of column of flow while his features spalled off at the edges, still screaming because the stuff was designed to hold its substratum together pretty stably while infiltrating and extracting. (Its proper role was for trash mining) And do I need to say it was between me and the way out? Definitely not to be recommended... Wnt (talk) 00:52, 2 March 2012 (UTC)
Luckily, my substance is not designed to self-replicate, and cannot function without the correct chromatic sequence. A series of finely timed pulses of particular intensities, and frequencies, causes the component molecules of the substance to structurally rearange themselves like a tiny rubix cube. Without the correct photochromatic signiture, the substance remains inactive. Plasmic Physics (talk) 01:31, 2 March 2012 (UTC)
Iridium windows ever really used in teletherapy devices to treat cancer?
[edit]The Wikipedia article on the Goiânia accident in Brazil in 1987 makes repeated mention of a radiation source holder that has an "iridium window" built into it, a window which is eventually "broken" releasing radioactive cesium chloride into the environment. The International Atomic Energy Agency's own report (here: http://www-pub.iaea.org/MTCD/publications/PDF/Pub815_web.pdf} mentions such a window many times. Problem is, such an item does not appear to exist, and I have been unable to find any citation in a book, academic or professional journal, or web site that mentions the use of iridium as a "window" in conjunction with radiation therapy outside of this single incident in Brazil. I have reviewed the original patent for the device (here: http://www.patsnap.com/patents/view/US3588031.html) and the authors make no mention of such a window. Nor has any patent issued since ever mentioned such a window. And I do not understand how this is possible-- not a single article? Not a line in a single book? Anywhere? Except in Brazil... in 1987... once. I am mystified and am hoping someone, anyone, can show me something about the use of iridium as a window for radiation teletherapy outside of this single incident. I am not used to coming up quite so empty handed. KDS4444Talk 11:54, 1 March 2012 (UTC)
- Kind of an interesting question. I don't really see very many technical specifications of the window materials in most sources. This IAEA publication notes lots of windows but only occasionally identifies the material in some models (e.g. beryllium). --Mr.98 (talk) 14:37, 1 March 2012 (UTC)
- Iridium being dense is likely to be less window like than other materials to radiation. Graeme Bartlett (talk) 09:55, 3 March 2012 (UTC)
Baryons, Quarks & the Pauli Exclusion Principle
[edit]Near the top of page: http://en.wiki.x.io/wiki/List_of_baryons it says: "Baryons composed of one type of quark (uuu, ddd, ...) can exist in J = 3⁄2 configuration, but J = 1⁄2 is forbidden by the Pauli exclusion principle". I must be missing the point here, because I thought that the inclusion of the colour of the quark as an additional quantum number avoided difficulties with Pauli. So what factor of a triple up quark violates Pauli if each quark is a different colour? As a follow-up question, I would ask if I am correct to assume that the total angular momentum, J, of a baryon is 3/2 if all the quarks are spinning in the same direction and 1/2 if one is pointing in the opposite direction from the other two? If so, then I would have expected a Pauli violation to be more likely when J=3/2 rather than when J=1/2. [I may have asked this question yesterday, but I cannot now find a copy and must assume that it disappeared into the ether!] Mrt47 (talk) 12:01, 1 March 2012 (UTC)
- The short answer is that, according to Pauli Exclusion Principle, the barion should be antisymmetric under the exchange of any two quarks. The ground state (angular momentum 0) of uuu is symmetric in space, symmetric in flavor, antisymmetric in color, and therefore it must be symmetric in spin. So it must have J=3/2. --Itinerant1 (talk) 23:37, 1 March 2012 (UTC)
Motorola RAZR V3i - Voicemail Problems
[edit]I have two questions about a second-hand Motorola RAZR V3i that I've got that I would like help with please.
Q.1 - When ever I get a voice message & try to listen to it again, the recording says "Press option 4 to listen again" but when I do press option 4 to listen the recording asks me to select an option & lists all the options again. It doesn't matter how many times I press option 4, I can't listen to my messages again & the recording just keeps repeating the options. Am I doing something wrong ?
Q.2 - When I've gone to various phone shops & asked them about this they keep teling me that I,ve got to go into the phones settings or options & "Set them properly". And some have even asked me why the No. 1 Key doesn't have the Voicemail symbol on it (the one that looks like to circles joined by a line either on their top or bottom), as they say "Should be as it's on all phones".
Scotius (talk) 12:12, 1 March 2012 (UTC)
- This is the science helpdesk. Consider posting this in the computer section. http://en.wiki.x.io/wiki/Wikipedia:Reference_desk/ComputingZzubnik (talk) 13:24, 1 March 2012 (UTC)
What makes a plant able to withstand extreme cold? Won't any water in it freeze and expand? XPPaul (talk) 12:20, 1 March 2012 (UTC)
- Take a look at antifreeze protein. Another tactic found in many alpine plants is to grow dense clusters of small individuals. This is an evolutionary response - any individual growing above the general population will be much more exposed to frost. SpinningSpark 12:46, 1 March 2012 (UTC)
- Evergreen conifer trees like pine and spruce have very narrow leaves and oily sap that stops them from freezing. "Many of them seasonally alter their biochemistry to make them more resistant to freezing, called "hardening"." Alansplodge (talk) 01:29, 2 March 2012 (UTC)
- Note that all these defenses can fail, due to extreme cold and other mitigating factors. See e.g. exploding tree. SemanticMantis (talk) 02:25, 2 March 2012 (UTC)
- Another strategy is to have the portion above the frost line die back, and leave behind a portion below the frost line, such as roots or a bulb, from which the plant will regrow the next year. This can be combined with the above strategy by having the portion below ground be frost-proof, as well. StuRat (talk) 05:08, 2 March 2012 (UTC)
Gravitational vs Relativistic time dilation
[edit]Is the time dilation for a non-accelerating observer on the surface of a massive body equal to that of an observer in free space travelling at said massive body's escape velocity (with respect to a third observer)? Goodbye Galaxy (talk) 14:42, 1 March 2012 (UTC)
- I'm fairly certain that is true: One of the central tenets of general relativity is the non-distinguishability of acceleration due to gravity compared to other forms of acceleration. The way I have heard it explained is that if you place a person in a windowless box, they would be unable to do any experiment or test to determin if an acceleration they experience is due to, say, a giant rocket on the outside of the box, or if it is because the box is sitting on the ground, and is experiencing the force of gravity. This is my understanding from reading physics as a "lay" person, someone with a less secular view may be able to provide more information. --Jayron32 14:56, 1 March 2012 (UTC)
- Thanks! Also, thank you Universe for being consistent (and awesome). Goodbye Galaxy (talk) 15:01, 1 March 2012 (UTC)
- (edit conflict) I just found a bit more. See Inertial_frame#General_relativity and Equivalence principle. --Jayron32 15:10, 1 March 2012 (UTC)
- Hold on, that example does not describe the situation the OP gives. You can't distinguish between gravity and constant acceleration, but he is talking about escape velocity. Yoenit (talk) 15:04, 1 March 2012 (UTC)
- (after EC reply to Yoenit) I'm not so sure on the mathematics of the situation he describes, so I'm not sure of the exact equivalence, but in principle, the concept that gravity is not a 'privileged' form of acceleration is basically a core part of GR. --Jayron32 15:10, 1 March 2012 (UTC)
- Fully agree, but an observer traveling at escape velocity is not accelerating, so that analogy does not hold. I was initially unsure whether the escape velocity was indeed the correct velocity, but you can see the equations given in Gravitational time dilation are identical to those for relativistic time dilation after you substitute in the escape velocity. The universe is indeed consistent and awesome. Yoenit (talk) 15:40, 1 March 2012 (UTC)
- (after EC reply to Yoenit) I'm not so sure on the mathematics of the situation he describes, so I'm not sure of the exact equivalence, but in principle, the concept that gravity is not a 'privileged' form of acceleration is basically a core part of GR. --Jayron32 15:10, 1 March 2012 (UTC)
- Thanks! Also, thank you Universe for being consistent (and awesome). Goodbye Galaxy (talk) 15:01, 1 March 2012 (UTC)
Cherry blossom front - formula
[edit]Hello, I have just created Cherry blossom front and it contains the following:
"The forecast is based on the Arrhenius equation, with the formula DTS = exp {9.5 × 103 × (t- 288.2) / (t× 288.2)}, where mean temperature is in Kelvin and DTS represents the number of days transformed to standard temperature."
Is this expressed correctly/clearly? (The formula is from here (in Japanese) and here's a related English-language abstract)
Thanks, Maculosae tegmine lyncis (talk) 16:00, 1 March 2012 (UTC)
- I've changed the formula for you. I'm not a native English speaker so I'll leave the grammar to the other editors. Anonymous.translator (talk) 16:22, 1 March 2012 (UTC)
Somewhat. Scientific convention is generally to use capital "T" for temperature, and "×" signs are generally not used outside expressing scientific notation common:
You could also write this as:
Depends really what you want to highlight when you display the formula. LukeSurl t c 00:17, 2 March 2012 (UTC)
- Thanks both, Maculosae tegmine lyncis (talk) 15:45, 2 March 2012 (UTC)
- Note that for simplicity of calculation and display you could write (approximately) Rich Farmbrough, 16:06, 2 March 2012 (UTC).
- Note that for simplicity of calculation and display you could write (approximately) Rich Farmbrough, 16:06, 2 March 2012 (UTC).
- Thanks both, Maculosae tegmine lyncis (talk) 15:45, 2 March 2012 (UTC)
In a PnP BJT transistor, the base, or the n is connected to both a negative terminal and a positive one. How can it be so?
[edit]I know that the p-n or the base emitter junction is forward biased and the base-collector is reverse biased for a BJT to operate. In order for this to be true, the sandwiched n layer has to be connected to a negative terminal of a source. In the second portion, or base collector n-p part, the sandwiched layer is again connected to a +ve terminal of a second voltage source. So, what would the potential actually be at the n junction terminal? — Preceding unsigned comment added by 210.4.65.52 (talk) 16:09, 1 March 2012 (UTC)
- The base is biased so that it is inbetween the collector and emitter bias voltages. It is perfectly possible for the base to be more negative than the emitter and more positive that the collector at the same time. See bipolar transistor biasing. SpinningSpark 16:56, 1 March 2012 (UTC)
Joints - not medical advice, just curiosity
[edit]Hello everyone! Short and sweet:
- Why do joints sometimes crackle or make sounds, i. e. like the crackling sound in knee joints when a person is squatting?
- What causes the swelling of joints i. e. after trauma? Where does the liquid in the joints come from (other than the normal fluid that is usually there)?
This is really just curiosity, I guess it might require a diagnosis based on Kainaw's criterion, but I'll leave that up to you. Thanks in advance. --Ouro (blah blah) 17:54, 1 March 2012 (UTC)
- For #1, see the oddly titled article Cracking joints. For #2, see Edema which is a general overview of swelling in the human body. In the case of an injury, the swelling is often part of inflamation, which is a very complex process. A brief overview of the swelling process itself is covered at Inflamation#Exudative_component. --Jayron32 18:09, 1 March 2012 (UTC)
- And today's word-I-didn't-know-before-but-now-resolve-to-use-in-conversation-as-often-as-possible is: Crepitus - Cucumber Mike (talk) 20:04, 1 March 2012 (UTC)
- Thanks. I'll read the articles You suggested (Jayron, I was just too tired last night... now I feel dumb that the title is that simple) and get back to You if I have any subsequent questions. --Ouro (blah blah) 13:06, 2 March 2012 (UTC)
- And today's word-I-didn't-know-before-but-now-resolve-to-use-in-conversation-as-often-as-possible is: Crepitus - Cucumber Mike (talk) 20:04, 1 March 2012 (UTC)
Genetic recombination
[edit]How common is chromosomal crossover in animal reproduction? Specifically, in the DNA of a typical human, have all 22 autosome pairs have been recombinated? Or is it usually only two or three autosome pairs? Or something different? Mathew5000 (talk) 21:03, 1 March 2012 (UTC)
- Centimorgan gives the average probability of a crossover as 1% over every million base pairs. Chromosome 1 is nearly 250 million base pairs long; chromosomes 21 and 22 are less than 50 million base pairs. Roughly speaking, at least one crossover is fairly likely (and multiple crossovers reasonably probable) in the longest chromosomes, and possible (but not guaranteed) in the shortest chromosomes. TenOfAllTrades(talk) 21:17, 1 March 2012 (UTC)
- Thank you, TenOfAllTrades. Mathew5000 (talk) 17:51, 3 March 2012 (UTC)




