Wikipedia:Reference desk/Archives/Science/2011 November 12
| Science desk | ||
|---|---|---|
| < November 11 | << Oct | November | Dec >> | November 13 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
November 12
[edit]Magpies
[edit]Why do magpies like to steal shiny objects? --70.250.212.95 (talk) 01:09, 12 November 2011 (UTC)
- Note that this behavior is observed in members of the crow family (Corvidae), not magpies alone. And corvids in general are remarkable for their intelligence that rivals that of primates. The behavior is known as neophilia, the attraction to new and interesting things. Juvenile corvids already exhibit this in play behavior, wherein they will pick up and play with interesting objects before eventually hiding it.
- Neophilia is related to food gathering. It's common in innovative omnivores (including us humans) as it helps in assessing the food sources of an environment. Short answer - curiosity. -- Obsidi♠n Soul 12:03, 12 November 2011 (UTC)
What raises metabolic rate?
[edit]Does anaerobic resistance training raise basal metabolic rate more than aerobic exercise? So many conflicting reports based on which sites you read. Aerobic sites think their way is best; weight lifting sites think their way will do the most for metabolic rate. How can I know who is right? 24.62.245.13 (talk) 01:31, 12 November 2011 (UTC)
- Compare the BMI of the typical top marathon runner to that of the typical top weight lifter. Count Iblis (talk) 01:41, 12 November 2011 (UTC)
- No, don't do that. That could be a selective effect. Indeed, at the moment of performing the exercise, aerobic burns more calories and anaerobic burns more calories along the day. 88.14.195.138 (talk) 01:44, 12 November 2011 (UTC)
- Our article on Basal metabolic rate cites this study in the The American Journal of Clinical Nutrition and this study in the Journal of Applied Physiology (which both seem to be fairly neutral sources) on the topic. This book and this book also touch on the issue. This isn't my area of expertise, so I'll avoid offering a definite conclusion here. Mark Arsten (talk) 04:14, 12 November 2011 (UTC)
- The basic idea is that aerobic exercise by its nature (low intensity but long duration) tends to burn more calories while you're doing it, but has little impact on resting metabolism outside training. Anaerobic resistance training is high intensity but short duration so doesn't burn a lot of calories while you're doing it, however a long term effect of it is muscle growth, and possibly changed body composition. Since muscles require more energy than say fat stores even when at rest, then having bigger muscles generally means you will have an increased BMR. The impact of a changed BMR is rather more complex, but is also beyond the scope of your question. --jjron (talk) 14:15, 12 November 2011 (UTC)
- That's assuming muscles, organs and brain produce more heat than needed to keep the body temperature constant. If not, the metabolism will be increased to do so. So while your argument may be valid for people living in warm environments, it's not certain this would apply in general. The increased muscle mass may not make a difference because your body has to burn calories to stay at 37°C anyway. 84.197.183.188 (talk) 00:32, 14 November 2011 (UTC)
- The basic idea is that aerobic exercise by its nature (low intensity but long duration) tends to burn more calories while you're doing it, but has little impact on resting metabolism outside training. Anaerobic resistance training is high intensity but short duration so doesn't burn a lot of calories while you're doing it, however a long term effect of it is muscle growth, and possibly changed body composition. Since muscles require more energy than say fat stores even when at rest, then having bigger muscles generally means you will have an increased BMR. The impact of a changed BMR is rather more complex, but is also beyond the scope of your question. --jjron (talk) 14:15, 12 November 2011 (UTC)
Honestly, the short answer is "anything". A lot of the confusion comes from people with strong views as to which aspects of fitness are most desirable. Everyone seems to describe their chosen style of fitness as "best". Best for what? The studies on marathon sites will show that long duration aerobic exercise burns the most calories during exercise, gives you good lung capacity and endurance etc. Weightlifting sites will cite studies that show body composition changes, increased muscle mass and strength etc. A yoga practitioner will probably focus on balance and flexibility. At the end of the day, any form of exercise will increase your metabolic rate vastly compared to doing nothing at all. Trying to decide on the healthiest form of exercise is a backwards way of doing it that completely disregards psychological effects (you are far more likely to incorporate exercise into your lifestyle in the long term if you find a sport/activity that you love, as opposed to just checking into the gym a few times a week for your health.) Decide what you want to be good at, and then train for that. Teshmanesh (talk) 13:26, 14 November 2011 (UTC)
Big boned
[edit]It's a common excuse to say "I'm not fat, I just have big bones". Is there something about it, does some people have bigger and heavier skeletons than others? It's just that I have a BMI of 21, but people (doctors also) think that I'm heavily underweight. — Preceding unsigned comment added by MummitroldLinda (talk • contribs) 09:12, 12 November 2011 (UTC)
- My vet told me my cat was big boned... he was a Persian cross, and Persians can be quite big. We were worried he was too heavy (I couldn't lift him), but the vet examined him and "big boned" was his diagnosis! So it should be the same for humans. --TammyMoet (talk) 10:06, 12 November 2011 (UTC) Here's a guide for how to tell what size frame you have. --TammyMoet (talk) 10:08, 12 November 2011 (UTC)
- Yes and no. Yes, some people do have bigger, heavier skeletons than others, and can naturally carry more weight. Some simple common tests include measuring wrist circumference, elbow width, or to measure across the shoulders at the back from the furthermost bone processes, not the skin or fleshy tissue (basically from the shoulder joints, where you can feel your arm meeting the torso when you move it around). Since your bone structure will essentially be unaffected by increases in fat or muscle, these measurements should not really change for an individual once they reach full body size. For a given height however they can vary for different people, and will give an indication of whether someone is 'big-boned' or something else. But here's the rub. Someone who is big boned can and generally will carry more body weight, but won't necessarily look fat - in fact a true big boned person can usually get away with carrying more body fat (not just weight) than someone who is not big boned, without really showing it. So to get to your question, usually people who use that excuse are simply using an excuse; if they look fat they probably are fat. Regarding the second part of your question on BMIs, they are really quite a crude measurement, and I wouldn't place a lot of stock in them. You need to consider a lot more variables than that uses, and if you want to more accurately find out whether you are carrying excess fat you need better tools, such as skin-fold tests or immersion tests. Have a read of Body_mass_index#Limitations_and_shortcomings. --jjron (talk) 14:03, 12 November 2011 (UTC)
- As far as BMI, "underweight" has a very specific meaning with BMI. In the united states, less than 18.5 BMI is underweight. So, it is not medically possible to have a BMI of 21 and be underweight without a serious medical problem. Wikipedia is not the place to try and diagnose and treat any medical problem you may have. -- kainaw™ 02:27, 13 November 2011 (UTC)
- I didn't mean to say that I was underweight, but that I look underweight, and I'm not looking for a diagnosis, I just wondered — Preceding unsigned comment added by MummitroldLinda (talk • contribs) 06:24, 13 November 2011 (UTC)
- The correct response when someone states that they have big bones is "I didn't know bones jiggled like that." --Jayron32 02:36, 14 November 2011 (UTC)
Aniline napth-1-ol (or α-napthol) coupling reaction
[edit]What happens when we add α-napthol to aniline diazonium salt at 0-5 degree celcius? Is the product a dye? What is its IUPAC name? i have checked the wiki pages for "Diazonium salts", "azodyes", "1-napthol" ,"aniline" and found no answer. please help. can you give the mechanism as well if possible? — Preceding unsigned comment added by 120.56.176.148 (talk) 12:28, 12 November 2011 (UTC)
- AFAIR the aniline salt will undergo diazonium coupling with the naphthol at the latter's 4 position. As for whether the product is a dye, I don't remember off the top of my head, but considering that many azo compounds are dyes, it probably is one as well. 67.169.177.176 (talk) 20:46, 12 November 2011 (UTC)
- It is indeed a dye - the page you linked to called it Organol Brown N (CAS number 3651-02-3), apparently also called at times Solvent Brown 4, Hexatype Brown A, and Ohio Brown AA. Both 1-(Phenylazo)-4-naphthol and 4-phenyldiazenyl-naphthalen-1-ol (as well as other variants) are given as the systematic name [1]. If you start with 2-Naphthol, you get the slightly more well known Sudan I, which has its own article page. Buddy431 (talk) 01:55, 13 November 2011 (UTC)
thanks a lot guys. you really have made my day — Preceding unsigned comment added by 120.56.188.75 (talk) 12:31, 13 November 2011 (UTC)
Hot dog aroma lingering on a toothbrush for two months
[edit]Over September, I ate a lot of hot dogs. Since then I've noticed a distinct hot dog aroma whenever I use my toothbrush (without toothpaste). Does anyone have any idea what it is about hot dogs and their ingredients that might cause this to happen? Or at least, has anyone else experienced this, or something similar? Cheers. Vranak (talk) 13:46, 12 November 2011 (UTC)
- That fake hickory-smoke flavouring (liquid smoke?) they use for hotdogs (both the mechanically-recovered-meat kind and the soy-never-was-meat kind) seems very persistent; I've had it on pots that required several thorough washings to fully expunge. That said, I don't keep a toothbrush for longer than 2 months (and even then it gets a visit from Dr Bleach occasionally), and if one ever smelled like anything other than nothing it'd bin it immediately. -- Finlay McWalterჷTalk 13:57, 12 November 2011 (UTC)
Heat
[edit]Is heat synonymous with infrared radiation? --T.M.M. Dowd (talk) 14:24, 12 November 2011 (UTC)
No. Heat is synonymous with heat energy, whereas infrared radiation is a form of electromagnetic radiation (another form of energy). Heat energy causes infrared radiation through blackbody radiation, and the energy contained in infrared radiation can be absorbed by matter to increase its heat energy. The one is a property of matter, the other one is basically a kind of field. Phebus333 (talk) 15:01, 12 November 2011 (UTC)
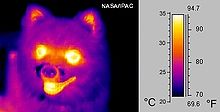
- It's a bit of a conceptual sticking-point because so many sources incorrectly use "infrared" and "heat" interchangeably. What you need to know is:
- Objects of any temperature emit blackbody radiation. For any object at Temperature T, electromagnetic energy is radiated at all wavelengths, specified by this equation - Planck's law.
- For any temperature "T", there is exactly one wavelength that is the "peak" blackbody wavelength - in other words, more energy is emitted at this wavelength than at any other wavelength. (Note that this is not equivalent to "most of the energy is emitted at this wavelength." This is a mathematical subtlety that you can verify for yourself, if you are pretty good with integral calculus). We can solve for the peak wavelength by playing with the Planck equation; the result is called Wien's displacement law and is a very easy formula to memorize.
- Because of the mathematical formalism, and because it makes for a very simple engineering task, we usually use the peak-radiated-wavelength to define the temperature; many types of machines rely on this phenomenon ("thermal infrared" cameras; certain types of laboratory thermometers, and so on). It so happens that if you solve Wien's displacement law for the temperatures that we commonly deal with - let's say, around 200 - 400 KelvinNote 1 (ranging from "slightly colder" to "slightly warmer" than the temperatures we think of as "comfortable") - you get wavelengths in the infrared band. So if we want to measure temperature, it's pretty easy to do so by measuring peak-wavelength; the machine we use happens to be an infrared spectrometer. Note that this is an indirect measure of temperature, and doesn't work for every case. (Try pointing an infrared temperature meter at a fluorescent light, for example - the result is completely wrong, because the radiation is due to fluorescence, not incandescence).
- (Note - more commonly, "infrared" refers to wavelengths ranging from ~750 nm all the way into the millimeter-wave radio band. Your definition may vary; so will your consequent temperature-range).
- For these reasons, a lot of sources use very flakey, semi-incorrect terminology - they often say "infrared radiation is emitted by hot objects" - while technically correct, this isn't really doing justice to the whole story. Nimur (talk) 18:23, 12 November 2011 (UTC)
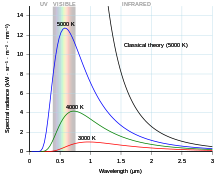
- I do not understand why integral calculus is necessary for an understanding of your second point. It is common sense: The standard distribution may average out at a certain value yet the bulk of the common value is elsewhere. See graph example at right. ~AH1 (discuss!) 19:18, 13 November 2011 (UTC)
- You can understand quite a bit of physics without ever trying to prove it! But if you want to prove certain statements, you need the rigorous formalism that usually takes the format of written mathematics. I found this very nice web page, "The Role of Mathematics in Physics", geared toward high-school level students. Nimur (talk) 19:55, 13 November 2011 (UTC)
- I do not understand why integral calculus is necessary for an understanding of your second point. It is common sense: The standard distribution may average out at a certain value yet the bulk of the common value is elsewhere. See graph example at right. ~AH1 (discuss!) 19:18, 13 November 2011 (UTC)
- Can the Kelvin be written in other SI units, can it be converted to Joules per kilogram? Or is it a unitless ratio, like the radian and the mole? Plasmic Physics (talk) 06:26, 14 November 2011 (UTC)
- There is of course a proportionality between the temperature and heat energy (in Joules) of a given object. Its called heat capacity. However, the kelvin itself is one of the 7 basic SI-units, and therefore not convertible into other SI-units. Phebus333 (talk) 17:46, 14 November 2011 (UTC)
- Can the Kelvin be written in other SI units, can it be converted to Joules per kilogram? Or is it a unitless ratio, like the radian and the mole? Plasmic Physics (talk) 06:26, 14 November 2011 (UTC)
- What I meant was, the other SI units can be defined in terms of other units e.g. kg = Pa m s2. What does one Kelvin mean? Plasmic Physics (talk) 03:21, 15 November 2011 (UTC)
- No. Actually, there are other units (like Pascal, Newton, etc.) which constitute a practical combination of some of the 7 basic SI-units, to allow for more convenient use under certain circumstances. The newton for example is kg*m*s-2, it would kinda suck if every measurement of force would have to be written with those units. However, this is all just a question of how we define our units for everyday use. I don`t think there are units derived from kelvin since the temperature (as a dimension) is not as fundamental as time or length (for example) which tend to appear in almost any physical formula.
- Oh, and for the radian and mole: the radian is in fact a dimensionless ratio, as it is defined as a certain fraction (or multiple) of your circles radius. The mole however is not a true dimensionless ratio, but rather a unit, as it is defined via the avogadro constant. One mole is 6.02214129×1023 molecules, so it has sort of a dimension ("pieces"/"number of particles"). The kelvin is not a unitless ratio, it is in itself a unit, just like the second or meter.
- I believe you are confusing different terms here: A unit is our way to measure differences in specific physical dimensions, which I believe could be explained as the basic structure of our space time. The term "unitless" is not really common among scientists, because every unit is associated to one (or, for derived units, several) physical dimensions, so the appropriate term would be dimensionless.
- And the question "what does one kelvin mean" (as I understand it) should be explained by its definition. If not, I`m afraid I do not understand your question. Phebus333 (talk) 12:48, 15 November 2011 (UTC)
- What I meant was, the other SI units can be defined in terms of other units e.g. kg = Pa m s2. What does one Kelvin mean? Plasmic Physics (talk) 03:21, 15 November 2011 (UTC)
- What I mean is: what does it mean to say that this object is warmer by 1 Kelvin than the other object - what is being quatified? Plasmic Physics (talk) 21:56, 15 November 2011 (UTC)
- The way I understand it, you really do quantify the temperature, via certain physical effects it has (like thermal expansion in an alcohol or mercury thermometer or blackbody radiation in a thermographic camera). Phebus333 (talk) 21:31, 16 November 2011 (UTC)
- What I mean is: what does it mean to say that this object is warmer by 1 Kelvin than the other object - what is being quatified? Plasmic Physics (talk) 21:56, 15 November 2011 (UTC)
- When two objects of different temperatures are brought into contact, there is a decelerating net flow of energy across the interface measured in J m-2 s-1. What is the rate of deceleration proportional except to the initial difference in temperature? Plasmic Physics (talk) 06:39, 14 November 2011 (UTC)
Why some proposed changes to SI base units?
[edit]For instance, the article says that the Consultative Committee for Units is considering redefining the metre from the distance traveled by light in a vacuum during 1/299792458 s to the magnitude you get by setting the speed c of light in a vacuum to exactly 299792458 m·s−1. Other than similarity in form with some of the other proposed definitions, what does this accomplish? A trivial drawback is that it's a bit indirect, requiring a bit of algebra to solve for a length.
I am no expert, but I do see a much more serious drawback in the philosophical notion underlying this definitional change. For this native speaker of English anyway, the connotation of the term "physical constant" clashes with the notion of defining to have some exact numerical value. Constants are constants, so we get to measure them, but we don't get to set them.
What do the experts in metrology have to say about this?—PaulTanenbaum (talk) 17:11, 12 November 2011 (UTC)
- Although we conventially say that c is a physical constant, it actually is nothing more than an irrelevant scaling constant. It is analogous to using different units to measure heat energy and kinetic energy. There would be then a conversion constant between the two units, nothing would stop you from assigning heat a different dimension than other forms of energy. But if you later realize that actually heat can also be measured in Joules, you may still be stuck with the convention that it is consered to be physically different and given an incompatible dimension. The SI conversion factor can then be defined to have some numerical value, you can also define natural units where the conversion factor is exactly 1. We ended up doing the latter in the SI system, but in case of the metre the situation is more similar to the former scenario.
- In the end we have to realize that physics is fundamentally dimensionless. Nature doesn't care about how we have chosen our unit system. Count Iblis (talk) 17:50, 12 November 2011 (UTC)
- Also note that there is no physical difference between the two definitions you describe. It's just a matter of different word choice. Semantics rather than physics. Dauto (talk) 20:22, 12 November 2011 (UTC)
- Length is more difficult to define in 'absolute' term than frequency. Thus, by this process mentioned, length & time can be defined by frequency. In other words, it is more accurate at defining these two fundamental units of measurement by something that is constant to oh, Something like 10 to the 18 or better.--Aspro (talk) 20:29, 12 November 2011 (UTC)
Of course Count Iblis's right about the scaling constant, but I think it misses my point. As to Dauto's comment, yes of course, it's semantics. That's why I talked about the connotation of the term "physical constant." The two forms of the definition end up effectively equivalent, since either may be transformed into the other by a trivial algebraic manipulation. The concerns that moved me to ask were merely that (1) talking about "defining a constant" seems to me odd and (2) defining a unit of length directly in terms of a length seems more straightforward than defining it so as to make a velocity come out just so. The issue on my latter concern has to do with the notion of defining. Here's a rough analogy: it seems better to define the baseball term battery as "the pitcher and the catcher" than as "the part of the team that includes none of the infielders or outfielders." The two definitions are effectively equivalent, but the former seems to do a better job of capturing the term's intension. That's all.—PaulTanenbaum (talk) 00:16, 13 November 2011 (UTC)
- Its not semantics! In the pitcher/catcher analogy, the two are never exactly the same distance apart throughout the game. The metre has (had) no fixed absolute length (and neither has the Kilogram).--Aspro (talk) 00:57, 13 November 2011 (UTC)
- PaulTanenbaum, I think the point you're missing is that the speed of light is indeed defined and the latter definition for the meter makes that point more clear. Dauto (talk) 01:03, 13 November 2011 (UTC)
- But Dauto, in what sense is the speed of light defined? I mean in what sense is it even susceptible to definition? Because it is a physical constant, which Wikipedia describes as "generally believed to be both universal in nature and constant in time," once one has defined speed and light, how is there any further choice in defining c? Of course its numerical value depends on the units in which one chooses to express it: mi/h, m/s, or whatever. But having chosen some units for velocity, we are then stuck with whatever value nature has ordained. And yes, it's clear that both the current definition of metre and its proposed replacement amount to defining the units for velocity in precisely the way that causes measurements of c to give the particular value 299792458. I just find it odd to say that one is defining a physical constant.
- I do understand what it means when people say things like, "To simplify the arithmetic, let's normalize our units so that c = 1." But for me, that doesn't read as a defining of c. Instead, it reads as a partial defining of the units of length and time—as a constraint C1 on the relationship between the units of length and time. Together with one more constraint C2 that nailed down either of those two units, the constraint c = 1 would then have the result of defining whichever of time and length had not been directly nailed down by C2. Under the pair {C1, C2} of constraints you end up with units for velocity that are light-years/year, or something similar.
- So what have I not yet understood?—PaulTanenbaum (talk) 03:33, 13 November 2011 (UTC)
- For a measurement standard to be useful as a standard, it needs to be available to everyone. When the metric system was being defined, there was a reasonably precise natural time standard that was measurable by anyone (the length of a solar day), but there were no obvious natural sources for other units, so they fell back on making a standard kilogram and two scratches on a standard metal bar, and then making copies of those masters, and copies of the copies... to distribute to everyone who needed to use the standard. Fortunately, the speed of light in vacuum is now easily measurable in any laboratory, so we can throw away the metal bar and its many copies and use the speed of light instead. The speed of light has different units than distance, but that doesn't matter; speed, time, and mass is a perfectly good set of base units. The speed of light is now 299792458 m/s by definition just as the weight of the standard kilogram is 1 kg by definition. Does that answer your question? -- BenRG (talk) 05:32, 13 November 2011 (UTC)
- Just to be pedantic – what is the universally agreed 'fundamental' definition of a Kilogram? New research promises a better way. NPL graphene research highlighted in Nature. Stick a Standards Lab in very, very high Earth obit (well a way from gravitational disturbances) and we (or more probably the Chinese) can have time, length, mass, ect., standards to a very high order -based all on fundamentals.--Aspro (talk) 17:04, 13 November 2011 (UTC)
- PaulTanenbaum, when people say "To simplify the arithmetic, let's normalize our units so that c = 1." they really mean c = 1 with no units attached. In other words, they are defining 1 light-year = 1 year, and velocity becomes a unitless physical quantity. Dauto (talk) 13:35, 13 November 2011 (UTC)
- The standard kilogram. Does the recent discovery of the OPERA neutrino anomaly have any bearing upon the speed of c = 1? ~AH1 (discuss!) 19:12, 13 November 2011 (UTC)
OK, Dauto, you wrote above that "they are defining 1 light-year = 1 year," i.e., that they are equating a distance and a duration. Do you mean that because distance and duration are both all rolled up into the single entity of space-time, they are therefore commensurable? In any event, what has that to do with the definability of physical constants?—PaulTanenbaum (talk) 19:51, 13 November 2011 (UTC)
- Yes, commensurability is an important factor here (Ultimately all physical quantities are commensurable and that's why Count Iblis said "In the end we have to realize that physics is fundamentally dimensionless"). Following Count Iblis' lead, let's take the more familiar heat vs work example. Work as other forms of energies is measured in Joules. Heat on the other hand was traditionally measured in calories. Work can be converted into heat which allows us to write the equation Q=kW where Q is the heat, W is the work, and k is a conversion factor k=(1/4.184) cal/J. We can also define k=1 in which case you get 1 cal= 4.184 J. The point we are trying to make is that c just as k is simply an irrelevant conversion factor between two different units. Dauto (talk) 20:48, 13 November 2011 (UTC)
Lemme try this differently. In what sense is it better to define metre (a unit of length) thus:
- D1—the magnitude you get by setting the speed of light in a vacuum to exactly 299792458 m·s−1
than to define it thus:
- D2—the distance traveled by light in a vacuum during 1/299792458 s?
As I see it, definiens D2 has the advantage that it defines this unit of length directly in terms of a distance, whereas definiens D1 (the proposed replacement for D2) gets at length only indirectly, by way of velocity. Now, you are free to argue that that advantage is negligible. OK, maybe it is. My only point is that I don't understand any advantage to D1. Sorry if I'm seeming obtuse, I just don't get how D1 is an improvement.—PaulTanenbaum (talk) 21:15, 13 November 2011 (UTC)
- Consistency - and don't overlook at what that consistency tells you about the redefinitions. If you look at the New SI definitions, you'll see they're all set up that way. Units are set to fix some physical constant to an exact number. For the metre, there's no real practical change, but for the other units (like the kilogram or the ampere) there's a real fundamental change on how those units are defined. The reason it seems minor for the metre is that the metre was the vanguard of this change - redefining the metre in terms of the speed of light instead of a physical object or the length of some physically realizable object (even the nebulous "physically realizability" of a wavelength of light) paved the way for the conceptual switch in which units are tied to fundamental constants of the universe, rather than some physical description of a measurement apparatus. It's a bold move by CIPM (well, bold by the standards of stodgy standards organizations) - we're saying that we have enough confidence in the constantness, invariability and and to some extent the "fundamentalness" of these constants that we are willing to base our measurement system on them. More so than just a practical change on how one measures a "kilogram" or an "ampere" in the lab, it's a subtle conceptual change in how we view these units in relation to the physical world (probably better stated as a recognition of the way they've come to be viewed in modern physics). At this point units aren't just an external measure imposed from without on the natural world, but are tied to and spring forth intrinsically from the fabric of the universe. That's what the redefinition of the metre is doing. It doesn't change how long a meter is either practically or theoretically, but it changes the concept itself of what it means to be an SI unit. -- 71.35.99.151 (talk) 23:10, 13 November 2011 (UTC)
- Think I've got my head around PaulTanenbaum question. By defining the metre as exactly 1⁄299 792 458 it fixes the length, not only better than a bar of platinum (try measuring a few hundred kilometres with one of those without getting compound errors) but also any where else in the universe – for all time. In interferometry, one can use monochromatic laser to measure physical objects in terms of 'wavelength' but these lasers are not truly monochromatic. The advantage of the current definition is, that as we improve lasers and other interferometric techniques, the length of metre does not change – instead we can determine its length more accurately. Doing it the other way around, the unit (length of the metre) is always on the move. We would have to keep redefining it with every advance in metrology/physics. Does that make sense?--Aspro (talk) 00:35, 14 November 2011 (UTC)
- I don't get the question. As the referred article states in the lede, the SI units are "arbitrary" definitions; the "physical constants" are not being expressed by some numerical value, it's the arbitrary unit that is defined with this number. 84.197.183.188 (talk) 00:51, 14 November 2011 (UTC)
- The way I read it the proposed definition also indirectly defines the second while the current definition is dependent on an existing different definition of the second. Roger (talk) 09:09, 15 November 2011 (UTC)
baldness and buzzcuts
[edit]Why when someone buzzes his head, you can see some dark points, but when someone is really bald, you can see a bright skin? — Preceding unsigned comment added by 77.125.152.76 (talk) 21:24, 12 November 2011 (UTC)
- Because when he has a buzzcut you can see the dark hair ends, but someone who's bald has nothing but bright, shiny scalp. Whoop whoop pull up Bitching Betty | Averted crashes 21:56, 12 November 2011 (UTC)
After you shave your head, your head is smooth, I speak about a razor-shave. You see a grey scalp, and not a white one. — Preceding unsigned comment added by 77.125.152.76 (talk) 22:53, 12 November 2011 (UTC)
- You can still see the ends, just like with blackheads. Add up a lot of them, and they make the scalp grey. StuRat (talk) 23:00, 12 November 2011 (UTC)
- If you haven't had a shaved head anytime recently and you have a skin tone that tans, you will see much lighter skin where the hair used to be. In places where this happens frequently (ie: Boot Camp or Military School), new recruits are easily identified by their shiny head. -- kainaw™ 02:21, 13 November 2011 (UTC)
- Hence the term "knobs", yes? ←Baseball Bugs What's up, Doc? carrots→ 07:54, 13 November 2011 (UTC)
- Knobs. Silver bullets. All places have a different name for it. -- kainaw™ 17:16, 13 November 2011 (UTC)
- Put simply, a naturally bald person is bald because they have no hair follicles. A person with hair still has these hair follicles, and if you shave the head these hair follicles are still present. As hair grows more or less constantly (except when it is in the resting phase of growth), the new growth will be visible. The article explains all this quite clearly. --TammyMoet (talk) 10:35, 13 November 2011 (UTC)
- But they asked about a just-shaven head, with no time for new hair growth. In this case, the cross sections of the cut hairs are still visible, and, since the skin is somewhat translucent, hair may be seen to some extent from the side, as well. StuRat (talk) 17:09, 13 November 2011 (UTC)
- Did he? That's not how I read it. --TammyMoet (talk) 19:18, 13 November 2011 (UTC)
- See also alopecia. ~AH1 (discuss!) 19:01, 13 November 2011 (UTC)
Is there a hair under the skin? if so, how long is it?
Another thing, if I will remove the whole hair but not the follicles, will my head be white or grey? By the way sometimes,when one of my hair falls, it has a white dot. What is this? — Preceding unsigned comment added by 77.125.152.76 (talk) 22:01, 13 November 2011 (UTC)
- If you used a tweezer or wax to rip all the hairs out down to the root, then I'd expect your scalp to lack the grey color, although it might be quite red due to all the irritation. Also, doing so repeatedly may make it difficult to regrow hair later. See hair follicle to find out what the "white dot" on the end is. StuRat (talk) 04:05, 14 November 2011 (UTC)
