Wikipedia:Reference desk/Archives/Science/2011 March 1
| Science desk | ||
|---|---|---|
| < February 28 | << Feb | March | Apr >> | March 2 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
March 1
[edit]I've read 3/4 of the article and still don't understand what the theory/idea is, other than it seeks to explain the Galaxy rotation curve. My question: Can someone explain MOND simply? Albacore (talk) 01:04, 1 March 2011 (UTC)
- It's a modification of Newton's theory of gravity in which the usual gravitational force law, G M m / r² = m a, is replaced by G M m / r² = m a μ(a/ao), where ao is a new constant and μ is tanh or another function with a similar shape. Does that help? -- BenRG (talk) 01:41, 1 March 2011 (UTC)
- Useful as if a classical limit that explains away the dark matter problem, then a relativistic field theory can be constructed with the relevant weak field limit. —Preceding unsigned comment added by 129.67.37.227 (talk) 10:24, 1 March 2011 (UTC)
- In popular science, MOND is stated as "That Newtons laws of gravitation does not hold over large distances, such as a galaxy". It introduces a fudge-factor into Newtons law of gravitation, which makes the law fit with observations of galaxy rotation. Adding invisible dark matter to the galaxy solves the problem too. The wikipedia article is a hard read but that's partly MONDs own fault. The theory does now answer the "why's" we are accustomed to in other theories, like "why would gravitation change?" It just states that this fudge factor works for the galaxy rotation problem. EverGreg (talk) 09:30, 2 March 2011 (UTC)
- The univers is cycla in time and at the age the arrow of time flip ' and it affect gravity , thanks water nosfim —Preceding unsigned comment added by 77.127.25.82 (talk) 10:36, 2 March 2011 (UTC)
How do oral ansthestics with benzocaine work?
[edit]Yes I have read the articles but I still dont understand how they work.Accdude92 (talk) 01:18, 1 March 2011 (UTC)
- There's a brief explanation at Benzocaine#Mechanism_of_action. Basically Benzocaine blocks the sodium ion channels that, when opened, propogate a signal down the nerves. Since the sodium channel stops working, no signals can get sent down the nerve. --Jayron32 01:34, 1 March 2011 (UTC)
- Then what makes the numbing fealing? If it cant transmit a signal, the why dont you feal nothing at all?Accdude92 (talk) 01:36, 1 March 2011 (UTC)
- It only blocks certain nerves, those which reach the surface of the skin and sense pain there. You also have nerves whose endings stop some distance under the skin; these nerves sense pressure but not touch. The "numb" sensation you feel when you use benzocaine comes from the fact that you've deadened the surface nerves, but not the deep nerves. Other "caines" like novocaine operate in the same manner; however they are usually injected subcutaneously, thus you tend to lose ALL sensation from the deadened region. With a topical anesthetic, however, you are only deadening the surface nerves. --Jayron32 01:58, 1 March 2011 (UTC)
- Then what makes the numbing fealing? If it cant transmit a signal, the why dont you feal nothing at all?Accdude92 (talk) 01:36, 1 March 2011 (UTC)
ear piercing
[edit]Is there ever a time when its impossible for an ear piercing to heal?Accdude92 (talk) 02:04, 1 March 2011 (UTC)
- Check out wound healing, it should work as with any wound. Constantly traumatizing the site can at least slow the process, also infection probably would do the trick.But frankly cases of non-healing wounds, I've heard about are rather discusting 84.52.32.149 (talk) 02:56, 1 March 2011 (UTC)
- Because I know girls that have left their earrings out for quite a while, and the piercing remained open.Accdude92 (talk) 03:25, 1 March 2011 (UTC)
- Oh, in that case it has acctualy healed i.e. there is no wound, but a miniature hole in ear, therefore I believe it can stay open indefinetly, though it usualy disapears 89.235.200.28 (talk) 03:37, 1 March 2011 (UTC)
- I dont understand how a piercing isnt a wound.Accdude92 (talk) 03:42, 1 March 2011 (UTC)
- Look at wound. A wound is when the skin (specifically, the dermis) is damaged. When an ear is pierced, there is initially a true wound: the skin is broken. Soon, though, skin regrows, not covering the hole (because theres an earing there), but rather around the inside of the hole. Compare it to, for example, losing a finger in an accident with a table saw. Initially there's a wound: skin is broken, and blood spurts everywhere. Soon, though, a clot forms, and a scab develops. Before too long, skin regrows, and the finger is the same as before, though a bit shorter, and with some possible scarring. The wound healing article really is pretty good. Buddy431 (talk) 04:08, 1 March 2011 (UTC)
- In other words, the tunnel through the earlobe is now lined with surface epithelium. DRosenbach (Talk | Contribs) 05:42, 1 March 2011 (UTC)
- It could be useful to look into process that causes holes to disapear in the first place. Perhaps wounding the site anew can cause it to close up (i.e. if skin is removed from within the hole and there is no earing, nothing would prevent wound from closing), I also figure that since something is inserted in ear initial wound could be healing little bit longer than normal, also if earrings are from material that person is alergic to could do the trick, especialy if alergic reaction is light and person dosen`t notice it right away, also removing and puting back earings might hurt the ear or if it hasn`t been moved at all it could grow into ear, which might cause new wound when removing the earing. So one might still have an unhealed wound by the time earings are removed for good. However I don`t think any of this is what normaly happens when these holes disapear, especialy if person has worn earings for long time 89.235.214.5 (talk) 11:34, 1 March 2011 (UTC)
- That would probably work. I have heard that you have to be careful with burn patients because if they have burns on their fingers and the fingers are not wrapped individually, they can sort of grow together when they heal. Googlemeister (talk) 17:25, 1 March 2011 (UTC)
- It could be useful to look into process that causes holes to disapear in the first place. Perhaps wounding the site anew can cause it to close up (i.e. if skin is removed from within the hole and there is no earing, nothing would prevent wound from closing), I also figure that since something is inserted in ear initial wound could be healing little bit longer than normal, also if earrings are from material that person is alergic to could do the trick, especialy if alergic reaction is light and person dosen`t notice it right away, also removing and puting back earings might hurt the ear or if it hasn`t been moved at all it could grow into ear, which might cause new wound when removing the earing. So one might still have an unhealed wound by the time earings are removed for good. However I don`t think any of this is what normaly happens when these holes disapear, especialy if person has worn earings for long time 89.235.214.5 (talk) 11:34, 1 March 2011 (UTC)
- In other words, the tunnel through the earlobe is now lined with surface epithelium. DRosenbach (Talk | Contribs) 05:42, 1 March 2011 (UTC)
- Look at wound. A wound is when the skin (specifically, the dermis) is damaged. When an ear is pierced, there is initially a true wound: the skin is broken. Soon, though, skin regrows, not covering the hole (because theres an earing there), but rather around the inside of the hole. Compare it to, for example, losing a finger in an accident with a table saw. Initially there's a wound: skin is broken, and blood spurts everywhere. Soon, though, a clot forms, and a scab develops. Before too long, skin regrows, and the finger is the same as before, though a bit shorter, and with some possible scarring. The wound healing article really is pretty good. Buddy431 (talk) 04:08, 1 March 2011 (UTC)
- I dont understand how a piercing isnt a wound.Accdude92 (talk) 03:42, 1 March 2011 (UTC)
The secret magic of color intensity?
[edit]I am taking an arts related course in university as an elective, it is meant for people studying audiovisual media. The teacher has been trying to make a point that something happens, if you put two colors of similar intensity together, but she can't explain what and when I tried to make her clerify this she said that I would see. What I see is that any two colors look difrent when put together, but frankly the particular combinations she sugests look dull. Also she seems to have discovered this effect on her own (she's a painter), so I can't find anything on this. What I want is someone to explain what effect this could posibly have 84.52.32.149 (talk) 02:45, 1 March 2011 (UTC)
- A color will look different juxtaposed to one color as opposed to another color. Neighboring colors cause the perception of each to be altered, vis-a-vis what would be the case if they each had different neighbors. Josef Albers did work in this area. Our article Munsell color system may be worth taking a look at. The terms used have to be defined. You refer to colors of "similar intensity". The term "intensity" doesn't appear in the Munsell article. But there may be a different term referring to that aspect of color. "Hue", "value", and "chroma" are terms used in that article. I'm not sure if one of those might be the approximate equivalent of "intensity". Bus stop (talk) 03:13, 1 March 2011 (UTC)
- For the record - she uses "similar lightness or darkness" 89.235.213.2 (talk) 03:30, 1 March 2011 (UTC)
- I believe that would be value in the Munsell system. Deor (talk) 03:44, 1 March 2011 (UTC)
- For the record - she uses "similar lightness or darkness" 89.235.213.2 (talk) 03:30, 1 March 2011 (UTC)
- In the absence of further information, it's difficult to interpret what, exactly, your teacher may be referring to; but the entries for "halation" and "vanishing boundary" here may be relevant. Deor (talk) 03:44, 1 March 2011 (UTC)
What makes this hard is that two colors that have equal luminance on one monitor may have different luminances when shown on a different monitor. See if this demo works for you. Looie496 (talk) 05:18, 1 March 2011 (UTC)
- What I learnt from experience, is that when two pigments of a given saturation are mixed, the resulting mixture is of less saturation than either of the constituent pigments.
- Just for clarification:
- Hue is the absolute colour on the spectrum.
- Saturation is a measure of how vivid the natural colour is, ranging from a dull grey to perfectly vivid.
- Luminosity is a measure of how bright the natural colour is, ranging from black to white.
- Just for clarification:
- Brown is equivalent to vivid (saturation) orange (hue), of low brightness (luminosity). Plasmic Physics (talk) 09:00, 1 March 2011 (UTC)
- She has been demonstrating by putting together solidly colored pieces of paper, rather than mixing paint. I think she means vanishing boundry, thanks, now I know what I am supposed to look for 89.235.214.5 (talk) 11:44, 1 March 2011 (UTC)
- This (the mixing problem) is explored on the level of particles of pigment in a book called Blue and Yellow Don't Make Green. 81.131.65.79 (talk) 12:38, 1 March 2011 (UTC)
- Brown is equivalent to vivid (saturation) orange (hue), of low brightness (luminosity). Plasmic Physics (talk) 09:00, 1 March 2011 (UTC)
I did this with silly putty once. I bought the bright neon green and pink (came together in a 2-pack) and of course ended up mixing them together. To my amazement the resulting color was the regular old dull-skin peach color of the original silly putty! They "cancelled" each other out. —Preceding unsigned comment added by 165.212.189.187 (talk) 13:53, 1 March 2011 (UTC)
- 84.52.32.149, you might wish to ask at the following online reference desk.
- —Wavelength (talk) 15:51, 1 March 2011 (UTC)
GPS suction cup problem
[edit]I've had three or four automobile GPS units, all different brands, and I've had the same problem with all of them. The suction cup works fine until it gets hot - then it won't stay on but a few minutes. (This is even when the inside of the car is cool.) If you wet the suction cup before sticking on the windshield, it holds. But once it the weather gets hot then you can't remove it properly.
Is there a way to get the suction cups to work properly in a hot environment? Bubba73 You talkin' to me? 05:17, 1 March 2011 (UTC)
- (I think you have a typo in the last sentence of the first paragraph.) Suction cups work by having one part of the suction cup "pull" against a different part of the suction cup. If the pull is not strong enough it falls. So what happens in the heat is that the rubber softens which reduces how springy it is, and it doesn't pull. What you need is one of those suction cups that have a mechanical lever to press it, instead of just relying on the elasticity of the rubber. Or find one with very very stiff rubber which will hopefully stay elastic even in the heat. Ariel. (talk) 06:08, 1 March 2011 (UTC)
- But the suction cup has to be in the mount for the GPS, so I don't see any way to change it. Bubba73 You talkin' to me? 06:25, 1 March 2011 (UTC)
- By typo I meant: In one case you say it falls off in hot weather, then you say you can't remove it in hot weather? Isn't that what you want? Anyway, you may be able to buy an aftermarket alternative GPS holder. Either one specifically for your model, or a generic type. Ariel. (talk) 06:55, 1 March 2011 (UTC)
- When it is new, it works. It holds it on until I want to take it off. But once the weather gets hot, it falls off every few minutes. Years ago someone suggested wetting the suction cup before putting it on. But once that is done and it is out in the heat, I can't get it off properly (it sticks to the windshield). I want it to stay on until I want to take it off. I take it off to move to the other car or to hide it. Bubba73 You talkin' to me? 14:11, 1 March 2011 (UTC)
- My Dad has a beanbag like thing that is glued (with a weak non-marking adhesive) to the dashboard of his car. It's intended to hold things like satnavs, glasses, and phones. He just puts his satnav down on this, and the satnav is held pretty effectively in place (due to the saggy nature of the bag and the clingy fabric). When he's done, he can return the satnav to the glove compartment just by lifting it off. It's proven to be much more successful than a variety of dashboard and screen-mounting suction cups. -- Finlay McWalter ☻ Talk 13:02, 1 March 2011 (UTC)
- Can we assume that you are also cleaning the windscreen and cup surface with alcohol to remove any greasy film. Greasy fingerprints (even if you can't see them) attracts dust which provides an easier access for air to creep in between the two surfaces. This is what a liquid film stops, but it also, as you have found, makes it very difficult to brake the air tight seal again. Also a little trapped air is going expand as the temperature rises so you might still have to re-stick if you first apply it in cooler conditions. On a different application, I stuck a disc of reflective film (just half again as big) on the outside and that helped enormously. But you must keep both surfaces clean!--Aspro (talk) 14:38, 1 March 2011 (UTC)
- No I have not been cleaning the surfaces. I'll try cleaning the glass with a glass cleaner and the suction cup with alcohol. Bubba73 You talkin' to me? 17:02, 1 March 2011 (UTC)
Physical Examination and Vital Signs
[edit]Is "pulse oximetry" and "oxygenation" one and the same thing as far as the patient's vital signs on physical examination are concerned? — Preceding unsigned comment added by Aniketnik (talk • contribs) 09:47, 1 March 2011 (UTC)
- Yes, probably. But there is also Arterial blood gas which is related but not the same. Ariel. (talk) 10:36, 1 March 2011 (UTC)
- From practical stand point, most likely. However any -metry means mesuring something, rather than the stuff being messured, in this particular case pulse pulse oximetry is used to messure oxygenation, and note that the article on it says that it is not just any kind of oxygenation, but oxygenation in hemoglobin 89.235.214.5 (talk) 12:01, 1 March 2011 (UTC)
Phenobarb level
[edit]What is the normal phenobarb level in a patient? What are the effects of a high phenobarb level in a patient, in what way does it affect the patient's health? — Preceding unsigned comment added by Aniketnik (talk • contribs) 10:07, 1 March 2011 (UTC)
- Assuming you mean Phenobarbital, then the normal level is zero. It's a drug, it's not naturally in a patient. Do you mean what is the typical therapeutic level? If so, then I don't know. For high doses see Phenobarbital#Overdose and Barbiturate overdose. Ariel. (talk) 10:32, 1 March 2011 (UTC)
Incomputable things.
[edit]Title is a bit unclear, so here we go:
British mathematician Alan Turing proved that there are true statements in mathematics that are incomputable, meaning beyond the reach of computers, no matter how new and powerful, how can it be explained that limited human mind came to know this statements?Intuition? Is Human intelligence something apart, never to be reached for machines?
- TY(again)
- DST —Preceding unsigned comment added by 194.138.12.167 (talk) 10:30, 1 March 2011 (UTC)
- I think you mean Gödel's incompleteness theorem not Undecidable problem. They are related but not the same. A barebones answer to your question is that we know it, but can't prove it. So that means while it seems correct whereever we check, we can't know that it's correct everywhere. This section and Mechanism (philosophy)#Gödelian arguments talk about it as well. Ariel. (talk) 10:44, 1 March 2011 (UTC)
- It is true that there are incomputable functions, but we, humans, can not compute them either. 157.193.175.207 (talk) 10:51, 1 March 2011 (UTC)
- so that's a red link, just look here http://en.wiki.x.io/wiki/Uncomputable_function#Incomputable_functions_and_unsolvable_problems 157.193.175.207 (talk) 10:52, 1 March 2011 (UTC)
I meant , that if such things cannot be logically proven , how did the human mind(intelligence) understand the concept? should it not be not understandable then? Is human intelligence something never to be reched by machines, something apart? DST —Preceding unsigned comment added by 194.138.12.167 (talk) 11:43, 1 March 2011 (UTC)
- The statement "uncomputable or undecidable statements exist" is not itself an uncomputable or undecidable statement. It can be "reached" by machines. 157.193.175.207 (talk) 13:04, 1 March 2011 (UTC)
- The entire trick here is that humans have wonderful abilities to express illogical statements. Human language has no requirement for being self-consistent (ergo the common graffito in college men's rooms: "This sentence is false."). Computability/completeness/etc. all require you to express things that are logically self-consistent. It's not so much that the human intelligence is "something apart," so much as we define machine intelligence as something logically consistent. Such is my meager understanding of these things, anyway. --Mr.98 (talk) 12:41, 1 March 2011 (UTC)
- No, that's not quite it. Gödel proved that for any formal system that is powerful enough to describe peano arithmetic, there is a statement that is true, but not provable in the formal system. We can, however, step outside the system and prove (some of) these statements on a meta-level. Of course, our meta-system will have its own unprovable truths, and so on. But in general, a proof of something does not have to be constructive. I can prove that some x with property P exists without being able to produce the x. This frequently happens with with a proof by contradiction, where I assume no such x exists and show that this leads to nonsensical conclusions. --Stephan Schulz (talk) 14:36, 1 March 2011 (UTC)
- Turing himself addressed this objection, which he called the Mathematical objection, in his famous essay Computing Machinery and Intelligence, as follows:
| “ | The short answer to this argument is that although it is established that there are limitations to the powers of any particular machine, it has only been stated, without any sort of proof, that no such limitations apply to the human intellect. But I do not think this view can be dismissed quite so lightly. Whenever one of these machines is asked the appropriate critical question, and gives a definite answer, we know that this answer must be wrong, and this gives us a certain feeling of superiority. Is this feeling illusory? It is no doubt quite genuine, but I do not think too much importance should be attached to it. We too often give wrong answers to questions ourselves to be justified in being very pleased at such evidence of fallibility on the part of the machines. Further, our superiority can only be felt on such an occasion in relation to the one machine over which we have scored our petty triumph. There would be no question of triumphing simultaneously over all machines. In short, then, there might be men cleverer than any given machine, but then again there might be other machines cleverer again, and so on. | ” |
- Looie496 (talk) 17:43, 1 March 2011 (UTC)
- Cantor's diagonal argument might be an interesting example of something similar. Basically it proves that all the irrational numbers can't be counted, if you can't count them all, how can you prove they can't be counted? This is as opposed to natural numbers which even though there's an infinite number of them CAN be counted. It would take you an infinite amount of time but you could count all the natural numbers, however even if you count for an infinite amount of time, you'll never count all the irrational numbers. :)Vespine (talk) 02:40, 2 March 2011 (UTC)
Contorting the human body
[edit]The image of the woman at this page is confusing me. (Note: Image is of a woman bent over backwards. She's clothed in a... something... but the image may not be entirely work safe) It's really the hump between her legs that has my reviewing my basic knowledge of the human skeleton. I'm of two minds about it, really. If it's a Photoshopped image, then why would the 'shopper put in such a distended hump? But if it's real, what's causing the hump? Her coccyx? Someone care to enlighten me? Dismas|(talk) 10:59, 1 March 2011 (UTC)
- This will help. Scroll down a bit and you can see the original, which has been deleted from the source, but tineye still has a cache of the thumbnail. Ariel. (talk) 11:24, 1 March 2011 (UTC)
- I think it is called Mons pubis. Dauto (talk) 16:28, 1 March 2011 (UTC)
- And to answer more of the questions, the picture was distorted, as can be seen from the difference to the tineye original. We may never know why the shopper distended this part, but probably to draw extra attention to it, even if the original picture looked more comfortable to look at. (If you ever consider a contortionist to be comfortably comported). Graeme Bartlett (talk) 11:53, 3 March 2011 (UTC)
PhL, Ph1, Ph2
[edit]What are the PhL, Ph1, Ph2 settings on a microscope supposed to do? ---Seans Potato Business 11:10, 1 March 2011 (UTC)
- Which kind of microscope? My one doesn't. Plasmic Physics (talk) 11:53, 1 March 2011 (UTC)
- You might find Phase contrast microscopy ansewers all your questions--Aspro (talk) 14:56, 1 March 2011 (UTC)
- The article fails to mention that PhL is simply the phase-plate on the Left-hand side as you sit before the microscope. Usually, the plate for the lowest useful magnification goes here so that you always know where it is by touch, rather than having to move your head away to check where each plate is by sight all the time. You can then get in to a rhythm of moving around the specimen slide(s) and going up through the magnification range with ease. You choose phase-plates 1 & 2 to suit the whatever higher mag ranges your using.--Aspro (talk) 15:54, 1 March 2011 (UTC)
3 questions of the nature of electricity
[edit]What happens to a electron in a magnetic field and a magnet in a electric field? is there any difference between negative and positive charges? Why do interacting magnetic fields create electricity? — Preceding unsigned comment added by Lufc88 (talk • contribs) 17:02, 1 March 2011 (UTC)
- For the first question, see Lorentz force; the simplest answer is "it tends to move in circles". For the second, see C-symmetry; the answer is "yes, via the weak interaction". (If you don't include antimatter, then they are more different because of the differences between the proton and the electron.) For the third (assuming you mean "changing" by "interacting"), see The Maxwell–Faraday equation; I'm not sure there is a good "why" answer, though. --Tardis (talk) 17:58, 1 March 2011 (UTC)
- Tardis' answer is correct ("yes, via the weak interaction"), but I worry that a non-physicist will not understand what he means; so I will elaborate a bit. Negatively-charged electrons have an antimatter "counterpart," the positively-charged positron. Positively-charged protons have an antimatter counterpart, the negatively-charged antiproton. "For most purposes", these are "exactly the same particle but with opposite charge." They have identical mass and spin, and opposite charge. (Those are pretty much the only properties of an electron or proton). However - if you "zoom in" for a closer look and analyze the way that each particle/anti-particle experiences the Weak interaction, it is clear that they are not just equal-but-opposite-charge particles. Physicists call that property "violation of the charge-symmetry" (or "C-symmetry") by the weak interaction.
- This symmetry breaking is a very complicated and subtle effect; it leads some theoretical high-energy physicists to postulate that the Universe fundamentally has a preferred coordinate system - which is an altogether unappealing idea, in the context of the rest of modern physical theory. Nimur (talk) 21:41, 1 March 2011 (UTC)
- Oh, but please elaborate! I'm a great fan of a preferred coordinate system, but I don't see how to get there from symmetry breaking. Wnt (talk) 19:55, 2 March 2011 (UTC)
- Neither do I! I am a bit out of my area of expertise here. If I recall correctly, I think this was the subject of a SLAC public lecture I attended (a while back). This one, ANTIMATTER: What is it and where did it go? seems to be the title I recall, but the date seems wrong. I suppose we could do a thorough literature review: that Google Scholar search brings up some familiar-looking stuff. Nimur (talk) 22:15, 2 March 2011 (UTC)
- (Actually, the lecture I'm thinking about was probably Our Lopsided Universe: The Matter with Anti-Matter. Both lectures are provided for online viewing at the SLAC links I provided. Nimur (talk) 22:37, 2 March 2011 (UTC)
- Neither do I! I am a bit out of my area of expertise here. If I recall correctly, I think this was the subject of a SLAC public lecture I attended (a while back). This one, ANTIMATTER: What is it and where did it go? seems to be the title I recall, but the date seems wrong. I suppose we could do a thorough literature review: that Google Scholar search brings up some familiar-looking stuff. Nimur (talk) 22:15, 2 March 2011 (UTC)
- Oh, but please elaborate! I'm a great fan of a preferred coordinate system, but I don't see how to get there from symmetry breaking. Wnt (talk) 19:55, 2 March 2011 (UTC)
- I neglected to answer the second half of the first question: nothing significant happens to a magnet in an electric field, although I wouldn't be surprised if the electric polarization of the atoms produced some small effects on their magnetism. --Tardis (talk) 17:34, 2 March 2011 (UTC)
Are phosphate esters chiral?
[edit]
Are phosphate esters (R1, R2, R3 all different in the image to the right) chiral? If so, are natural organophosphates enantiopure or racemic? —Preceding unsigned comment added by 148.177.1.215 (talk) 18:04, 1 March 2011 (UTC)
- Some phosphate esters are chiral, see page 4 (labelled p. 28) of this for example, but it depends on what groups the Rs are as to whether it is or not. I can't find a source for your second question, since information about nature is drowned out by chemical information, but I would guess that they will be enantiopure, since compounds that are synthesised by enzymes have to be. SmartSE (talk) 22:17, 1 March 2011 (UTC)
- Thanks, that's a useful document. 148.177.1.215 (talk) 22:42, 1 March 2011 (UTC)
- Yes, as long as there are two R group that are unique, it is chiral. Plasmic Physics (talk) 22:29, 1 March 2011 (UTC)
- Don't you mean three R groups need to be unique? With only two different groups, it will have a plane of symmetry and therefore not be chiral, right? 148.177.1.215 (talk) 22:42, 1 March 2011 (UTC)
- Well, but if two of R1, R2, R3 are unique, can the third be a duplicate? ;) Wnt (talk) 22:44, 1 March 2011 (UTC)
- It could, but the P wouldn't be a stereocenter. DMacks (talk) 13:40, 2 March 2011 (UTC)
- Well, but if two of R1, R2, R3 are unique, can the third be a duplicate? ;) Wnt (talk) 22:44, 1 March 2011 (UTC)
- In general it is possible to isolate and study enantiomers,[1] though I'm not sure what the rate of inversion actually is. There has been some study of chiral phospholipids in biological systems. Wnt (talk) 22:43, 1 March 2011 (UTC)
- Rate of spontaneous inversion seems fairly low for all sorts of XYZP=O structures...what's more interesting is the same is true even for XYZP: (unlike tertiary amines, which undergo pyramidal inversion easily). (CH3)(t-Bu)(Ph)P ΔG‡=136.9 kJ/mol at 130 °C. Chiral phosphonyl chlorides undergo clean inversion in SN2 reactions. The geometry is pretty solid--ligands don't migrate while bound to the P center--but rather the racemization for alkoxy-substituted P appears to be generally by an acid-catalyzed ligand dissociation/reattachment (SN1-like) or other multistep mechanisms involving planar intermediates. (ref: ISBN 0854042466 §3.2.11 "Racemization, Enantiomerization and Diasteriomerization: Silicon, Phosphorus and Sulfur Compounds"). One extreme example that demonstrates intrinsic stability of the oxy (and halo and amino...) phosophorus center is the configurational stability (and study-ability and multistep reaction sequences involved with) monoalkyl phosphates. CH3OPO3H2 is a rock: the protons can dissociate/reassociate (no control over which two O are formally OH and which is =O) but the O do not come off or invert at the P center. DMacks (talk) 03:13, 2 March 2011 (UTC)
- Thanks DMacks. That is very helpful. 148.177.1.215 (talk) 16:08, 2 March 2011 (UTC)
- Rate of spontaneous inversion seems fairly low for all sorts of XYZP=O structures...what's more interesting is the same is true even for XYZP: (unlike tertiary amines, which undergo pyramidal inversion easily). (CH3)(t-Bu)(Ph)P ΔG‡=136.9 kJ/mol at 130 °C. Chiral phosphonyl chlorides undergo clean inversion in SN2 reactions. The geometry is pretty solid--ligands don't migrate while bound to the P center--but rather the racemization for alkoxy-substituted P appears to be generally by an acid-catalyzed ligand dissociation/reattachment (SN1-like) or other multistep mechanisms involving planar intermediates. (ref: ISBN 0854042466 §3.2.11 "Racemization, Enantiomerization and Diasteriomerization: Silicon, Phosphorus and Sulfur Compounds"). One extreme example that demonstrates intrinsic stability of the oxy (and halo and amino...) phosophorus center is the configurational stability (and study-ability and multistep reaction sequences involved with) monoalkyl phosphates. CH3OPO3H2 is a rock: the protons can dissociate/reassociate (no control over which two O are formally OH and which is =O) but the O do not come off or invert at the P center. DMacks (talk) 03:13, 2 March 2011 (UTC)
- Don't you mean three R groups need to be unique? With only two different groups, it will have a plane of symmetry and therefore not be chiral, right? 148.177.1.215 (talk) 22:42, 1 March 2011 (UTC)
Weak interaction: help
[edit]As to my previous questions have alluded, I am attempting to improve the Weak interaction page. (A vital article.) I've pretty much got what was there to how I'd want to be; however, if there isn't more to add, I'm the Queen of Sheba. So perhaps you have something to add or improve? Thanks. Grandiose (me, talk, contribs) 20:27, 1 March 2011 (UTC)
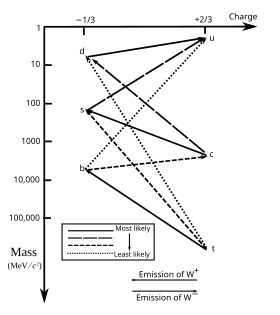
- Queen of Sheba? I don't get it. That Article needs improvement. That picture from the article for instance is wrong. Dauto (talk) 21:45, 1 March 2011 (UTC)
- In short, I'm not the Queen of Sheba. I've corrected the image. If you have any suggestions for the article, I'll act on them as best I can. Grandiose (me, talk, contribs) 22:01, 1 March 2011 (UTC)
- The picture is still not right. The line connecting b and c should be solid and so should the line connecting s and u. Dauto (talk) 00:43, 2 March 2011 (UTC)
- The diagram is adapted from information presented by Stanford, and as such could we find a (better) source to suggest the change? Grandiose (me, talk, contribs) 17:43, 2 March 2011 (UTC)
- That's a sloppy job done by whoever did it in Stanford. For instance the s-quark can only decay to a u-quark. No other decays are allowed. So that decay must be characterized as very likely (100% chance in fact). Dauto (talk) 19:54, 2 March 2011 (UTC)
- No, as the likelihoods are for a specific interval, not for an infinite one. s to u is a slower rate decay than those listed as very likely. —Preceding unsigned comment added by 129.67.37.227 (talk) 20:26, 2 March 2011 (UTC)
- The text than needs to be change to reflect that, because that's not what is written in the caption. Dauto (talk) 18:44, 3 March 2011 (UTC)
- No, as the likelihoods are for a specific interval, not for an infinite one. s to u is a slower rate decay than those listed as very likely. —Preceding unsigned comment added by 129.67.37.227 (talk) 20:26, 2 March 2011 (UTC)
- That's a sloppy job done by whoever did it in Stanford. For instance the s-quark can only decay to a u-quark. No other decays are allowed. So that decay must be characterized as very likely (100% chance in fact). Dauto (talk) 19:54, 2 March 2011 (UTC)
- The diagram is adapted from information presented by Stanford, and as such could we find a (better) source to suggest the change? Grandiose (me, talk, contribs) 17:43, 2 March 2011 (UTC)
- The picture is still not right. The line connecting b and c should be solid and so should the line connecting s and u. Dauto (talk) 00:43, 2 March 2011 (UTC)
Short English People
[edit]Everyone knows that the English people (and presumably other peoples) used to be short a few hundred years ago. I have never seen any published article regarding this, however it is reinforced whenever you visit someone’s quaint 15th century cottage and hit your head on the 5 foot ceilings, or walk round a country house and notice a suit of armour is particularly diminutive.
What caused this sudden increase in height, or alternatively, what caused the stunting in growth?
I have often heard that it was because of either a) diet or b) childhood disease c) starting work earlier.
If diet, what did they lack or were being poisoned by? Surely at least some parts of society was well fed but it seems to across all classes (citation needed) 92.28.85.122 (talk) —Preceding undated comment added 21:58, 1 March 2011 (UTC).
- Nutrition accounts for a huge amount of height. See Human_height#Determinants_of_growth_and_height for a lot of discussion of this. It isn't "poison" so much as "you eat only one staple or two, don't get enough protein, are lacking several major vitamins." Note that it's not necessarily whole life nutrition — nutrition as a child can affect overall height by a huge factor, even if later you end up with a more improved diet.[2] --Mr.98 (talk) 22:11, 1 March 2011 (UTC)
- But surely most or many people were not malnourished even if they did not have a huge excess of calories?, especially those who lived in the country, on farms etc (i.e. where these tiny cottages are. My impression is that people over 5”4 are almost unknown.
- Are there any studies that show when this sudden increase in height happened and what specifically was added, i.e. more protein, vitamins etc?
- Oh, another question. Are these deficiencies pre-natal or in early childhood? Thanks. 92.28.85.122 (talk) —Preceding undated comment added 22:19, 1 March 2011 (UTC).
- Yes, most people were malnourished, at least during part of their childhood, by modern standards (back then they would likely have said that if you didn't die, your diet was adequate). No, people over 5'4" were not almost unknown. The range of heights was similar then to what it is now, although the average was shorter. Protein is likely the major factor, as poor people rarely got meat, then, and didn't know how to get all the amino acids needed for their bodies to build proteins without meats, with combos of nuts, beans, and grains. Deficiencies which affect adult height could be prenatal, early childhood, or late childhood, up to when adult height would have been reached. Also note that there would be a class difference, with upper classes being the same height as modern people, due to getting plenty of protein (they might also have had gout and other diseases, from too much animal fat, BTW).StuRat (talk) 22:56, 1 March 2011 (UTC)
- While you may not have intended to suggest so, to avoid confusion I would note gout isn't caused by animal fat Nil Einne (talk) 15:04, 2 March 2011 (UTC)
- No, I meant that other diseases are caused by excess consumption of animal fat.StuRat (talk) 17:08, 2 March 2011 (UTC)
- While you may not have intended to suggest so, to avoid confusion I would note gout isn't caused by animal fat Nil Einne (talk) 15:04, 2 March 2011 (UTC)
- A recently observed case of this is Japan, where the traditional low-protein and low-fat diet has been influenced by western culture in the post World War II period. This table shows an average hieght increase of more than 5cm for 17 year-old boys between 1948 and 1980. This has been accompanied by a legion of negative health issues discussed here. Alansplodge (talk) 10:54, 4 March 2011 (UTC)
- I think we should consider that easy availability of food may lead to increased height and body weight in subsequent generations by an epigenetic mechanism of acquired inheritance, including changes in the methylation state of IGFBP3 and other factors in the IGF and growth hormone pathway.[3][4] These changes in methylation are reversible, but could become permanent over time by transitions from methylcytosine to thymidine. (Compare Cope's law) Wnt (talk) 17:49, 4 March 2011 (UTC)
Eating a diamond
[edit]can sucking or eating a diamond can kill a human being, how much average mass of diamond can kill a normal human and why.--True path finder (talk) 22:52, 1 March 2011 (UTC)
- It's not going to dissolve and release toxic chemicals, so the only dangers are mechanical:
- 1) If large enough, it could cause a blockage. This would be a multi-million dollar diamond.
- 2) If the cut has sharp edges, it could cut the walls of the digestive tract, leading to infection, etc.
- So, unless it's huge or sharp, I'd expect it to pass through without a problem. As for sucking on a diamond, I see no problem with that, as long as it's sterilized first. If it has some rather deadly microbes on it, that could be a problem, of course. StuRat (talk) 22:58, 1 March 2011 (UTC)
- You might also want to read our article on the syndrome called pica. Most often, pica is associated with mental health issues. Nimur (talk) 01:37, 2 March 2011 (UTC)
- Eating an intact diamond is unlikely to do any harm. Eating a small quantity of diamond that has been ground to powder can be fatal, though -- it gets into the walls of the digestive tract (as mentioned above) and causes bleeding. (Full disclosure -- I don't know for certain that this actually works. I learned about it from the Autobiography of Benvenuto Cellini, who is not an entirely reliable source.) Looie496 (talk) 01:56, 2 March 2011 (UTC)
- Snopes says "no", not that they're an entirely reliable source. --Sean 19:33, 2 March 2011 (UTC)
- Well, that source says that ground glass is harmless - though I wonder about ground lead glass! What would you call that, LithargeXT® Time Release Capsules? Anyway, I suspect the ground diamond would be harmless, but you'd have to do the experiment to know. See also Carbon nanotube#Toxicity, though of course, that's another thing again. Wnt (talk) 20:50, 2 March 2011 (UTC)
- Snopes says "no", not that they're an entirely reliable source. --Sean 19:33, 2 March 2011 (UTC)
If the diamond's owner finds out she'll kill you. So, yeah. Short Brigade Harvester Boris (talk) 08:18, 5 March 2011 (UTC)
Centre of the Universe
[edit]Just wanted to pose a question which has been puzzling me. Apparently galaxy UDFj-39546284 is the most astronomical object/galaxy ever to have been observed from earth, which lies 13.2bn light years away from us. Now assuming that scientists are correct in their assertion that the big bang occured 13.7bn years ago, then I assume it is theoretically impossible to observe anything beyond the range of 13.7bn lightyears from earth. Now here is my question... if we are able to observe a galaxy 13.2bn LY away from earth does that mean that we must be situated pretty near to the centre of the universe in order to see it? Or could it be that we are 6.6bn lightyears east of the centre of the universe observing something which might be 6.6bn lightyears west of the centre? Or is there even a centre at all? I understand there is a fair chance that the answer to my question might be that we just dont know! —Preceding unsigned comment added by 79.74.240.134 (talk) 22:59, 1 March 2011 (UTC)
- The metric expansion of space enters heavily into this sort of thing -- while the Horrendous Space Kablooie happened less than 14 bn years ago, expansion means that our theoretical horizon for observation is something like 40 bn light years, even though we'd only be seeing things 13 bn years old or so at that distance. As for the "center" -- it's everywhere. The Big Bang encompassed the entire observable universe, and it's expanded to everything we can now observe (and all but certainly plenty that we can't). The cosmic microwave background radiation is an incredibly strong piece of evidence supporting this -- in all directions, we observe the remnants of the Big Bang, and the intensity is the same in all directions. We are at the center. But if we measured it 10 bn light years distant, we'd be at the center there, too. — Lomn 23:23, 1 March 2011 (UTC)
- In other words, there is no center. Dauto (talk) 00:47, 2 March 2011 (UTC)
- The center is like, everywhere, and nowhere. Heavy, dude. Say, you gonna bogart that thing all night? :-) --Trovatore (talk) 00:49, 2 March 2011 (UTC)
- I actually lolled.Vespine (talk) 01:26, 2 March 2011 (UTC)
- The center is like, everywhere, and nowhere. Heavy, dude. Say, you gonna bogart that thing all night? :-) --Trovatore (talk) 00:49, 2 March 2011 (UTC)
- In other words, there is no center. Dauto (talk) 00:47, 2 March 2011 (UTC)
- I found the centre of the Universe, its in the sock which I lost in the dryer. Now if only I could find that sock... Plasmic Physics (talk) 04:04, 2 March 2011 (UTC)
- The answer is 42. Roger (talk) 09:48, 2 March 2011 (UTC)
Remember, the Big Bang isn't something you could "watch" from the outside. Most people think that if you were present at the time, you could take a box of popcorn and SPF 10100 suntan lotion and watch the world go boom. The fact is, there was no "Outside", as the entire universe, including the fabric of space (and time) was scrunched up in a tiny ball. So the center is everywhere. ManishEarthTalk • Stalk 12:05, 2 March 2011 (UTC)
- (EC)Exactly, so all those CG representations of the Big Bang, where everything starts off black and then you see a big explosion of flames and particles (similar to the Death Star exploding), just ain't accurate. For a start, even if there was a TV camera present at the time, it would have no way of being outside the 'explosion' beforehand - there was no space outside. It could not be viewed from outside. The explosion was more of a rapid expansion, and may have been viewable (theoretically with the right hot-suit) from the inside (the centre), but that's what we're doing now - viewing the explosion from the inside (the centre), just with no need for a hot-suit. --KägeTorä - (影虎) (TALK) 12:45, 2 March 2011 (UTC)
- That is also the reason why questions that contain the phrase "before the Big Bang" are totally meaningless. Roger (talk) 12:42, 2 March 2011 (UTC)
A little note: If you were to measure the speeds of all galaxies from Earth, it would appear that they are all moving away from us, which would make it apparent that we are the "center of the universe". But, this will happen on any galaxy'. This is because, if you view the universe as a rubber sheet, with dots representing galaxies, stretching the sheet would best describe the motion of the galaxies, as here all intergalactic distances get magnified, and everything moves away from everything ManishEarthTalk • Stalk 12:56, 2 March 2011 (UTC)
- If the curvature of space is negative, it was a tiny sphere, and is now a huge sphere. If it's zero, it was a plane, exactly the same size and shape as what we have now i.e. infinite, though it stretched out. If it was positive, it was infinite, but the curvature was crazy high as you approach the start, so a ball with a radius of one meter could have a volume on the order of cubic lightyears. — DanielLC 17:39, 3 March 2011 (UTC)
Technically speaking, the universe is multiple dimensions (I don't remember the exact number - 10 or 11 maybe?) in a 1 dimensional space. Tis 1 dimensional space is called the "Multiverse". In that regard, it cannot have a center, as its laws of physics only apply to it, and nowhere else. Therefore, as observed from the outside of this universe, our universe is simply a "membrane", or "brane" for short, that folds in on itself countless times. If you COULD observe from an outside position, which is impossible for two reasons: 1 - photons don't exist "outside", and 2 - the concept of mass doesn't exist "outside", (so you would either cease to exist, or die in some way so horrific that it can't be described in modern physics), you would see that any and every point is the "center" because any point is "0", with 0 being on each side of the universe, and adding those up and dividing them by two gives you zero. Verba et Acta. -Aholb 14:41, 2 March 2011 (UTC) — Preceding unsigned comment added by Aholb (talk • contribs)
- This is all M-Theory stuff you're talking, which has no experimental backing at all. --Sean 19:39, 2 March 2011 (UTC)
It important to see the difference between the universe and the observable universe. We are in the centre of our observable universe. Every other point is also in centre of its observable universe. The universe could be anything from slightly smaller than the observable universe to infinitely large. We have no idea if the universe has a meaningful centre or were it is located if it exist. --Gr8xoz (talk) 20:56, 2 March 2011 (UTC)
