User talk:Esander311/sandbox
Possible Topics :
High importance Stubs: Adenylation http://en.wiki.x.io/w/index.php?title=Adenylylation
Chiasma http://en.wiki.x.io/wiki/Chiasma_(genetics)
Co-Transport http://en.wiki.x.io/wiki/Active_transport#Secondary_active_transport
Oogenesis http://en.wiki.x.io/w/index.php?title=Oogenesis
Mid-Importance Stubs:
Acetyl Co-A
http://en.wiki.x.io/w/index.php?title=Acyl%2DCoA
Calcium Storage http://en.wiki.x.io/wiki/Calcium_in_biology
DNA Polymerase II http://en.wiki.x.io/wiki/DNA_polymerase_II
Microtubule Nucleation http://en.wiki.x.io/wiki/Microtubule_nucleation
For the co-Transport page, we could add more specific examples of how co-transport is used in different cellular functions, and different molecules that utilize it. We could also add more scholarly article reference sources and possible design or find some graphics. We also can add information about different diseases that can be caused by mutations of cotransport.
Yang, Chao-Ling et al. “WNK Kinases Regulate Thiazide-Sensitive Na-Cl Cotransport.” Journal of Clinical Investigation 111.7 (2003): 1039–1045. PMC. Web. 4 Nov. 2017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC152590/
http://www.pnas.org/content/93/23/13367.short#cited-by
Hubner, Christian A. “Disruption of KCC2 Reveals an Essential Role of K-Cl Cotransport Already in Early Synaptic Inhibition.” Neuron, Cell Press, 7 June 2001, www.sciencedirect.com/science/article/pii/S0896627301002975. — Preceding unsigned comment added by Esander311 (talk • contribs) 02:17, 4 November 2017 (UTC)
History:
In 1997, Jens C Skous, a Danish Medical Doctor [1] received the Nobel Prize in Chemistry for his research regarding the Sodium Potassium pump [2]
One category of Co-Transporters that is especially prominent in research regarding diabetes treatment [3] is Sodium Glucose Co-Transporters. These transporters were discovered by scientists at that National Health Institute [4]. These scientists had noticed a discrepancy in the absorption of glucose at different points in the kidney tubule of a rat. The gene was then discovered for intestinal glucose transport protein and linked to these membrane sodium glucose cotransport systems. The first of these membrane transport proteins was named SGLT1 followed buy the discovery of SGLT2 [5].
Anti port after first sentence: This antiport mechanism is important within the membranes of cardiac muscle cells in order to keep the calcium concentration in the cytoplasm low. [6]
Details after plant sentence: For example, the molecules Chlorine (Cl^-) and Nitrate NO3- exist in the cytosol of plant cells, and need to be transported into the vacuole. While the vacuole has channels for these ions, transportation of them is against the concentration gradient, and thus movement of these ions is driven by hydrogen pumps, or proton pumps[7]
Article — Preceding unsigned comment added by Esander311 (talk • contribs) 01:23, 11 November 2017 (UTC)
Cellular Transportation Mechanisms
[edit]Active transport is the movement of molecules across a membrane from a region of their lower concentration to a region of their higher concentration—in the direction against some gradient or other obstructing factor (often a concentration gradient).
Unlike passive transport, which uses the kinetic energy and natural entropy of molecules moving down a gradient, active transport uses cellular energy to move them against a gradient, polar repulsion, or other resistance. Active transport is usually associated with accumulating high concentrations of molecules that the cell needs, such as ions, glucose and amino acids. If the process uses chemical energy, such as from adenosine triphosphate (ATP), it is termed primary active transport. Secondary active transport involves the use of an electrochemical gradient. Examples of active transport include the uptake of glucose in the intestines in humans and the uptake of mineral ions into root hair cells of plants.[8]
History
[edit]In 1848, the German physiologist Emil Heinrich du Bois-Reymond suggested the possibility of active transport of substances across membranes.[9]
Rosenberg (1948) formulated the concept of active transport based on energetic considerations,[10] but later it would be redefined.
In 1997, Jens C Skous, a Danish Medical Doctor [11] received the Nobel Prize in Chemistry for his research regarding the Sodium Potassium pump [12]
One category of Co-Transporters that is especially prominent in research regarding diabetes treatment [13] is Sodium Glucose Co-Transporters. These transporters were discovered by scientists at that National Health Institute [14]. These scientists had noticed a discrepancy in the absorption of glucose at different points in the kidney tubule of a rat. The gene was then discovered for intestinal glucose transport protein and linked to these membrane sodium glucose cotransport systems. The first of these membrane transport proteins was named SGLT1 followed buy the discovery of SGLT2 [15].
Background
[edit]Specialized transmembrane proteins recognize the substance and allow it to move across the membrane when it otherwise would not, either because the phospholipid bilayer of the membrane is impermeable to the substance moved or because the substance is moved against the direction of its concentration gradient.[16] There are two forms of active transport, primary active transport and secondary active transport. In primary active transport, the proteins involved are pumps that normally use the chemical energy in the form of ATP. Secondary active transport, however, makes use of potential energy, which is usually derived through exploitation of an electrochemical gradient. The energy created from one ion moving down it's electrochemical gradient is used to power the transport of another ion moving against it's electrochemical gradient [17]. This involves pore-forming proteins that form channels across the cell membrane. The difference between passive transport and active transport is active transport requires energy, and moves substances against their respective concentration gradient, whereas passive transport requires no energy and moves substances in the direction of their respective concentration gradient[18].
In an antiporter, one substrate is transported in one direction across the membrane while another is cotransported in the opposite direction. In a symporter, two substrates are transported in the same direction across the membrane. Antiport and symport processes are associated with secondary active transport, meaning that one of the two substances is transported against it's concentration gradient, utilizing the energy derived from the transport of another ion (mostly Na+, K+ or H+ ions) down its concentration gradient.
If substrate molecules are moving from areas of lower concentration to areas of higher concentration[19] (i.e., in the opposite direction as, or against the concentration gradient), specific transmembrane carrier proteins are required. These proteins have receptors that bind to specific molecules (e.g., glucose) and transport them across the cell membrane. Because energy is required in this process, it is known as 'active' transport. Examples of active transport include the transportation of sodium out of the cell and potassium into the cell by the sodium-potassium pump. Active transport often takes place in the internal lining of the small intestine.
Plants need to absorb mineral salts from the soil or other sources, but these salts exist in very dilute solution. Active transport enables these cells to take up salts from this dilute solution against the direction of the concentration gradient. For example, the molecules Chlorine (Cl^-) and Nitrate NO3- exist in the cytosol of plant cells, and need to be transported into the vacuole. While the vacuole has channels for these ions, transportation of them is against the concentration gradient, and thus movement of these ions is driven by hydrogen pumps, or proton pumps[20]
Primary active transport
[edit]
Primary active transport, also called direct active transport, directly uses metabolic energy to transport molecules across a membrane.[21] Substances that are transported across the cell membrane by primary active transport include metal ions, such as Na+, K+, Mg2+, and Ca2+. These charged particles require ion pumps or ion channels to cross membranes and distribute through the body.
Most of the enzymes that perform this type of transport are transmembrane ATPases. A primary ATPase universal to all animal life is the sodium-potassium pump, which helps to maintain the cell potential. The sodium-potassium pump maintains the membrane potential by moving three Na+ ions out of the cell for every two [22] K+ ions moved into the cell. Other sources of energy for Primary active transport are redox energy and photon energy (light). An example of primary active transport using Redox energy is the mitochondrial electron transport chain that uses the reduction energy of NADH to move protons across the inner mitochondrial membrane against their concentration gradient. An example of primary active transport using light energy are the proteins involved in photosynthesis that use the energy of photons to create a proton gradient across the thylakoid membrane and also to create reduction power in the form of NADPH.
Model of active transport
[edit]ATP hydrolysis is used to transport hydrogen ions against the electrochemical gradient (from low to high hydrogen ion concentration). Phosphorylation of the carrier protein and the binding of a hydrogen ion induce a conformational (shape) change that drives the hydrogen ions to transport against the electrochemical gradient. Hydrolysis of the bound phosphate group and release of hydrogen ion then restores the carrier to its original conformation.[23]
Types of primary active transporters
[edit]- P-type ATPase: sodium potassium pump, calcium pump, proton pump
- F-ATPase: mitochondrial ATP synthase, chloroplast ATP synthase
- V-ATPase: vacuolar ATPase
- ABC (ATP binding cassette) transporter: MDR, CFTR, etc.
Secondary active transport
[edit]
In secondary active transport, also known as coupled transport or co-transport, energy is used to transport molecules across a membrane; however, in contrast to primary active transport, there is no direct coupling of ATP; instead it relies upon the electrochemical potential difference created by pumping ions in/out of the cell.[24] Permitting one ion or molecule to move down an electrochemical gradient, but possibly against the concentration gradient where it is more concentrated to that where it is less concentrated increases entropy and can serve as a source of energy for metabolism (e.g. in ATP synthase). The energy derived from the pumping of protons across a cell membrane is frequently used as the energy source in secondary active transport. In humans, Sodium, (Na+), is a commonly co-transportd ion across the plasma membrane, who's electrochemical gradient is then used to power the active transport of a second ion or molecule against it's gradient [25]. In bacteria and small yeast cells, a commonly co-transported ion is hydrogen [26]. Hydrogen pumps are also used to create an electrochemical gradient to carry out processes within cells such as in the Electron transport chain, an important function of Cellular respiration that happens in the Mitochondrion of the cell[27]
In August 1960, in Prague, Robert K. Crane presented for the first time his discovery of the sodium-glucose cotransport as the mechanism for intestinal glucose absorption.[28] Crane's discovery of cotransport was the first ever proposal of flux coupling in biology.[29][30]
Cotransporters can be classified as symporters and antiporters depending on whether the substances move in the same or opposite directions.
Antiport
[edit]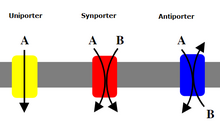
In an antiport two species of ion or other solutes are pumped in opposite directions across a membrane. One of these species is allowed to flow from high to low concentration which yields the entropic energy to drive the transport of the other solute from a low concentration region to a high one.
An example is the sodium-calcium exchanger or antiporter, which allows three sodium ions into the cell to transport one calcium out.[31] This antiport mechanism is important within the membranes of cardiac muscle cells in order to keep the calcium concentration in the cytoplasm low. [32] Many cells also possess calcium ATPases, which can operate at lower intracellular concentrations of calcium and sets the normal or resting concentration of this important second messenger.[33] But the ATPase exports calcium ions more slowly: only 30 per second versus 2000 per second by the exchanger. The exchanger comes into service when the calcium concentration rises steeply or "spikes" and enables rapid recovery.[34] This shows that a single type of ion can be transported by several enzymes, which need not be active all the time (constitutively), but may exist to meet specific, intermittent needs.
Symport
[edit]Symport uses the downhill movement of one solute species from high to low concentration to move another molecule uphill from low concentration to high concentration (against its concentration gradient). Both molecules are transported in the same direction.
An example is the glucose symporter SGLT1, which co-transports one glucose (or galactose) molecule into the cell for every two sodium ions it imports into the cell.[35] This symporter is located in the small intestines,[36] heart,[37] and brain.[38] It is also located in the S3 segment of the proximal tubule in each nephron in the kidneys.[39] Its mechanism is exploited in glucose rehydration therapy[40] This mechanism uses the absorption of sugar through the walls of the intestine to pull water in along with it.[41]. Defects in SGLT2 prevent effective reabsorption of glucose, causing familial renal glucosuria.[42]
Bulk Transport
[edit]Endocytosis and exocytosis are both forms of bulk transport that move materials into and out of cells, respectively, via vesicles.[43] In the case of Endocytosis, the cellular membrane folds around the desired materials outside the cell.[44] The ingested particle becomes trapped within a pouch, known as a vesicle, inside the cytoplasm. Often enzymes from lysosomes are then used to digest the molecules absorbed by this process.Substances that enter the cell via signal mediated endocytosis include proteins, hormones and growth factors.[45] Viruses enter cells through a form of endocytosis that involves their outer membrane fusing with the membrane of the cell. This forces the viral DNA into the host cell.[46]
Biologists distinguish two main types of endocytosis: pinocytosis and phagocytosis.[47]
- In pinocytosis, cells engulf liquid particles (in humans this process occurs in the small intestine, where cells engulf fat droplets).[48]
- In phagocytosis, cells engulf solid particles.[49]
Exocytosis involves the removal of substances through the fusion of the outer cell membrane and a vesicle membrane[50] An example of exocytosis would be the transmission of neurotransmitters across a synapse between brain cells.
See also
[edit]References
[edit]- ^ "Jens C. Skou - Biographical". Nobelprize.org. Nobel Media AB 2014. Web. 11 Nov 2017
- ^ "Jens C. Skou - Biographical". Nobelprize.org. Nobel Media AB 2014. Web. 11 Nov 2017
- ^ Inzucchi, Silvio E et al. “SGLT-2 Inhibitors and Cardiovascular Risk: Proposed Pathways and Review of Ongoing Outcome Trials.” Diabetes & Vascular Disease Research 12.2 (2015): 90–100. PMC. Web. 11 Nov. 2017
- ^ Story of Discovery: SGLT2 Inhibitors: Harnessing the Kidneys to Help Treat Diabetes.” National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Department of Health and Human Services, www.niddk.nih.gov/news/research-updates/Pages/story-discovery-SGLT2-inhibitors-harnessing-kidneys-help-treat-diabetes.aspx.
- ^ Story of Discovery: SGLT2 Inhibitors: Harnessing the Kidneys to Help Treat Diabetes.” National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Department of Health and Human Services, www.niddk.nih.gov/news/research-updates/Pages/story-discovery-SGLT2-inhibitors-harnessing-kidneys-help-treat-diabetes.aspx.
- ^ Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 15.6, Cotransport by Symporters and Antiporters. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21687/
- ^ Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 15.6, Cotransport by Symporters and Antiporters. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21687
- ^ "The importance of homeostasis". Science. me. Retrieved 23 April 2013.
- ^ Du Bois-Reymond, E. (1848–84). Untersuchungen über thierische Elektricität Berlin: Reimer. (Vol. 1, Part 1, 1848; Vol. 1, Part 2, 1849; Vol. 2, Part 1, 1860; Vol. 2, Part 2, 1884).
- ^ Rosenberg, T. (1948). On accumulation and active transport in biological systems. I. Thermodynamic considerations. Acta Chem. Scand. 2, 14-33, [1].
- ^ "Jens C. Skou - Biographical". Nobelprize.org. Nobel Media AB 2014. Web. 11 Nov 2017
- ^ "Jens C. Skou - Biographical". Nobelprize.org. Nobel Media AB 2014. Web. 11 Nov 2017
- ^ Inzucchi, Silvio E et al. “SGLT-2 Inhibitors and Cardiovascular Risk: Proposed Pathways and Review of Ongoing Outcome Trials.” Diabetes & Vascular Disease Research 12.2 (2015): 90–100. PMC. Web. 11 Nov. 2017
- ^ Story of Discovery: SGLT2 Inhibitors: Harnessing the Kidneys to Help Treat Diabetes.” National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Department of Health and Human Services, www.niddk.nih.gov/news/research-updates/Pages/story-discovery-SGLT2-inhibitors-harnessing-kidneys-help-treat-diabetes.aspx.
- ^ Story of Discovery: SGLT2 Inhibitors: Harnessing the Kidneys to Help Treat Diabetes.” National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Department of Health and Human Services, www.niddk.nih.gov/news/research-updates/Pages/story-discovery-SGLT2-inhibitors-harnessing-kidneys-help-treat-diabetes.aspx.
- ^ Active Transport Process. Buzzle.com (2010-05-14). Retrieved on 2011-12-05.
- ^ Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 15.6, Cotransport by Symporters and Antiporters. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21687/
- ^ Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Chapter 15, Transport across Cell Membranes. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21525/
- ^ Active Transport Archived August 24, 2011, at the Wayback Machine. Biologycorner.com. Retrieved on 2011-12-05.
- ^ Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 15.6, Cotransport by Symporters and Antiporters. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21687
- ^ Nosek, Thomas M. "Section 7/7ch05/7ch05p11". Essentials of Human Physiology. Archived from the original on 2016-03-24.
- ^ Reese, Jane B.; Urry, Lisa A.; Cain, Michael L.; Wasserman, Steven A.; Minorsky, Peter V.; Jackson, Robert B. (2014). Tenth Edition, Campbell's Biology. United States: Pearson Education Inc. p. 135. ISBN 978-0-321-77565-8 – via Tenth edition.
- ^ Cooper, Geoffrey (2009). The Cell: A Molecular Approach. Washington, DC: ASM PRESS. p. 65. ISBN 9780878933006.
- ^ Nosek, Thomas M. "Section 7/7ch05/7ch05p12". Essentials of Human Physiology. Archived from the original on 2016-03-24.
- ^ Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Carrier Proteins and Active Membrane Transport. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26896/
- ^ Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Carrier Proteins and Active Membrane Transport. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26896/
- ^ Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Electron-Transport Chains and Their Proton Pumps. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26904/
- ^ Crane, Robert K.; Miller, D.; Bihler, I. (1961). "The restrictions on possible mechanisms of intestinal transport of sugars". In Kleinzeller, A.; Kotyk, A. (eds.). Membrane Transport and Metabolism. Proceedings of a Symposium held in Prague, August 22–27, 1960. Prague: Czech Academy of Sciences. pp. 439–449.
- ^ Wright EM, Turk E (February 2004). "The sodium/glucose cotransport family SLC5". Pflügers Arch. 447 (5): 510–8. doi:10.1007/s00424-003-1063-6. PMID 12748858.
Crane in 1961 was the first to formulate the cotransport concept to explain active transport [7]. Specifically, he proposed that the accumulation of glucose in the intestinal epithelium across the brush border membrane was coupled to downhill Na+
transport cross the brush border. This hypothesis was rapidly tested, refined and extended [to] encompass the active transport of a diverse range of molecules and ions into virtually every cell type. - ^ Boyd CA (March 2008). "Facts, fantasies and fun in epithelial physiology". Exp. Physiol. 93 (3): 303–14. doi:10.1113/expphysiol.2007.037523. PMID 18192340.
p. 304. "the insight from this time that remains in all current text books is the notion of Robert Crane published originally as an appendix to a symposium paper published in 1960 (Crane et al. 1960). The key point here was 'flux coupling', the cotransport of sodium and glucose in the apical membrane of the small intestinal epithelial cell. Half a century later this idea has turned into one of the most studied of all transporter proteins (SGLT1), the sodium–glucose cotransporter.
- ^ Yu, SP; Choi, DW (June 1997). "Na(+)-Ca2+ exchange currents in cortical neurons: concomitant forward and reverse operation and effect of glutamate". The European Journal of Neuroscience. 9 (6): 1273–81. doi:10.1111/j.1460-9568.1997.tb01482.x. PMID 9215711.
- ^ Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 15.6, Cotransport by Symporters and Antiporters. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21687/
- ^ Strehler, EE; Zacharias, DA (January 2001). "Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps". Physiological reviews. 81 (1): 21–50. PMID 11152753.
- ^ Patterson, M; Sneyd, J; Friel, DD (January 2007). "Depolarization-induced calcium responses in sympathetic neurons: relative contributions from Ca2+ entry, extrusion, ER/mitochondrial Ca2+ uptake and release, and Ca2+ buffering". The Journal of General Physiology. 129 (1): 29–56. doi:10.1085/jgp.200609660. PMC 2151609. PMID 17190902.
- ^ Wright, EM; Loo, DD; Panayotova-Heiermann, M; Lostao, MP; Hirayama, BH; Mackenzie, B; Boorer, K; Zampighi, G (November 1994). "'Active' sugar transport in eukaryotes". The Journal of Experimental Biology. 196: 197–212. PMID 7823022.
- ^ Dyer, J; Hosie, KB; Shirazi-Beechey, SP (July 1997). "Nutrient regulation of human intestinal sugar transporter (SGLT2) expression". Gut. 41 (1): 56–9. doi:10.1136/gut.41.1.56. PMC 1027228. PMID 9274472.
- ^ Zhou, L; Cryan, EV; D'Andrea, MR; Belkowski, S; Conway, BR; Demarest, KT (1 October 2003). "Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT2)". Journal of cellular biochemistry. 90 (2): 339–46. doi:10.1002/jcb.10631. PMID 14505350.
- ^ Poppe, R; Karbach, U; Gambaryan, S; Wiesinger, H; Lutzenburg, M; Kraemer, M; Witte, OW; Koepsell, H (July 1997). "Expression of the Na+-D-glucose cotransporter SGLT1 in neurons". Journal of Neurochemistry. 69 (1): 84–94. doi:10.1046/j.1471-4159.1997.69010084.x. PMID 9202297.
- ^ Wright EM (2001). "Renal Na+-glucose cotransporters". Am J Physiol Renal Physiol. 280 (1): F10–8. PMID 11133510.
- ^ Loo, DD; Zeuthen, T; Chandy, G; Wright, EM (12 November 1996). "Cotransport of water by the Na+/glucose cotransporter". Proceedings of the National Academy of Sciences of the United States of America. 93 (23): 13367–70. doi:10.1073/pnas.93.23.13367. PMC 24099. PMID 8917597.
- ^ Loo, Donald D.; Zeuthan, Thomas; Chandy, Grischa; Wright, Ernest M. (1996-11-12). "Cotransport of water by Na+/glucose cotransporter" Proceedings of the National Academy of Sciences. 93 (23): 13367-13370. ISSN 0027-8424. PMID 8917597.
- ^ Wright EM, Hirayama BA, Loo DF (2007). "Active sugar transport in health and disease". Journal of internal medicine. 261 (1): 32–43. doi:10.1111/j.1365-2796.2006.01746.x. PMID 17222166.
- ^ Reece, Jane; Urry, Lisa; Cain, Michael; Wasserman, Steven; Minorsky, Peter; Jackson, Robert (2014). Tenth Addition Campbell Biology. United States of America: Pearson Education, Inc. p. 137. ISBN 978-0-321-77565-8 – via Tenth Addition.
- ^ Transport into the Cell from the Plasma Membrane: Endocytosis – Molecular Biology of the Cell – NCBI Bookshelf. Ncbi.nlm.nih.gov (2011-10-03). Retrieved on 2011-12-05.
- ^ Paston, Ira; Willingham, Mark C. (1985). Endocytosis. Springer, Boston, MA. pp 1-44. doi: 10.1007/978-1-4615-6904-6_1. ISBN 9781461569060.
- ^ Jahn, Reinhard, and Thomas C Sudhof. “Membrane Fusion and Exocytosis.” Membrane Fusion and Exocytosis | Annual Review of Biochemistry, Annual Review of Biochemistry, July 1999, www.annualreviews.org/doi/full/10.1146/annurev.biochem.68.1.863.
- ^ Cell : Two Major Process in Exchange Of Materials Between Cell And Environment Archived August 11, 2010, at the Wayback Machine. Takdang Aralin (2009-10-26). Retrieved on 2011-12-05.
- ^ Pinocytosis: Definition. biology-online.org
- ^ Phagocytosis. Courses.washington.edu. Retrieved on 2011-12-05.
- ^ Jahn, Reinhard, and Thomas C Sudhof. “Membrane Fusion and Exocytosis.” Membrane Fusion and Exocytosis | Annual Review of Biochemistry, Annual Review of Biochemistry, July 1999, www.annualreviews.org/doi/full/10.1146/annurev.biochem.68.1.863.
Notes
[edit]- Lodish H.; Berk A.; Zipursky S.L.; Matsudaira P.; Baltimore D.; Darnell J. (2000). "Section 15.6 Cotransport by Symporters and Antiporters". Molecular Cell Biology (4th ed.). New York: W.H. Freeman. ISBN 0-7167-3136-3.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)
External links
[edit]
