User:Nadkars1/sandbox
Article Evaluation
[edit]Stuff that I learned about writing an article[1]
Choose an article on Wikipedia related to your course to read and evaluate. As you read, consider the following questions (but don't feel limited to these):
- Is everything in the article relevant to the article topic? Is there anything that distracted you?
- I looked at the article about auxins. There is nothing that distracted me and it is relevant to my class material. I think that it articulated quite well the structure of auxins and how it functions in a multicellular environment.
- Is the article neutral? Are there any claims, or frames, that appear heavily biased toward a particular position?
- It is neutral and is not biased. It may leave out some minor history but it is not heavily biased towards one particular topic.
- Are there viewpoints that are overrepresented, or underrepresented?
- No
- Check a few citations. Do the links work? Does the source support the claims in the article?
- Yes, the sources work however some of the sources are books which is problematic because the pages linked in the book are not included in free previews of Google Books. This is a problem because it does not allow the reader to focus on the shortcomings of the article when describing the book.
- Is each fact referenced with an appropriate, reliable reference? Where does the information come from? Are these neutral sources? If biased, is that bias noted?
- Most of the sources are from academic journals or books. These are peer reviewed articles which have a strong basis in fact, and therefore are considered to be neutral sources.
- Is any information out of date? Is anything missing that could be added?
- No.
- Check out the Talk page of the article. What kinds of conversations, if any, are going on behind the scenes about how to represent this topic?
- Mostly unreferenced material
- How is the article rated? Is it a part of any WikiProjects?
- Wikiproject Plants, Wikiproject for Molecular and Cell Bioloy
- How does the way Wikipedia discusses this topic differ from the way we've talked about it in class?
- We did not talk about it yet but I assume it is more broad, while we will cover it in a narrower aspect. Wikipedia tells us everything but we are interested in seeing it as it relates to seed plants.
Look up 3-5 potential topics related to the course that you might want to update on Wikipedia. Review the content of the article and check the Talk page to see what other Wikipedians are already contributing. Identify one or two areas from each that you could improve.
There's not much in the Talk page, just a comment about paraphyly in the Carophycae page, but there are a lot of citations on that page. The pedicel and phytopharmacology page did not have much on the research and not many sources as well. I can improve the article of phytopharmacology using primary literature on how plants (such as marijuana and opioids). I can improve the charophycae articles by elaborating on their evolutionary characteristics. I can improve on the pedicel article by talking about the biochemistry and development of the stem.
Bibliography
[edit]Compile a list of relevant, reliable books, journal articles, or other sources. You need to find 10 sources that are from peer reviewed scientific sources.
[edit]Existing Phytopharmacology Article
[edit]Phytopharmacology is a term coined by the Russian scientist David Macht in the 1930s. Macht used the term for the field of study of the effects of drugs on plants. The term has since changed its meaning to become an established field of drug research, where the active substances come from plants (a field Macht would have called zoopharmacology where the drugs are applied to humans or animals). One journal in the field is Phytomedicine. The advantages of seeking medicines from plants are due both to the millions of years of co-evolution between plants and animals which has led to interactions between their constituent chemicals,[citation needed] and the nature of enzyme driven synthesis leading to optically pure chiral molecules whose reactions in the mammalian body can be very specific.
Many pharmacological preparations currently in use are derived from plants. Digoxin and aspirin are two of the earliest commercially refined plant preparations still available.
Extended Draft
[edit]What can be added to this article?
Overall: There is a lot of historical information on this page, however there is not enough about the actual compounds which are used in the practice of phytopharmacology. The page is mostly a definition of when phytopharmacology first arose in the literature and what distinguishes it from other medicinal uses of plants, such as herbalism. I want to expand this article by incorporating the different compounds which are used in phytopharmacology, as seen below and adapted from some of my sources[2]Most of the compounds that I will cover are secondary metabolites, which means that they are not the general compounds which are produced primarily for plant growth and development.
Glycosides are functional groups bonded to a sugar via a glycosidic linkage. They are a class of secondary metabolite which is bound to a mono- or oligo-saccharide or to uronic acid. There are many types of plant glycosides used in medicine; a brief description of the most prominent ones can be found below.[3]

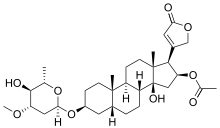
Cardiac Glycosides are a type of glycoside responsible for controlling the activity of Na+/K+ ATP-ase pumps in the cell membrane, and are able to treat atrial fibrillation in recent years and also very recently as a cancer therapy. They have seen when patients are taking drugs such as digitalis, digoxin, and oleandrin, all derived from Digitalis purpurea L. (foxglove), they have more benign cells and less recurrences of cancers.[4] These molecules are cytotoxic because they are able to induce an apoptotic pathway called Apo2L/TNF-related apoptosis-inducing ligand (TRAIL) in non-small-cell lung cancer cells by increasing the expression of death receptors 4 and 5.[5] Glucosinolates are associated with the Brassica family, these compounds are sulfurous and amino-acid derived compounds and act as anti-cancer agents.[2] They have a distinct taste and some have been identified as potent cancer-prevention agents (phase II detoxification enzymes, such as quinone reductase, glutathione-S-transferase, which is found in broccoli) and have also been able to treat some gastritis, caused by Heliobacter pylori. These metabolites have an malevolent effect on the thyroid because of their goitrogenic, or thyroid hormone inhibitory function, activity but it is not shown to have a significant impact on human health.[6] In fact, the World Cancer Research Fund found that the glucosinolates found in cruciferous vegetables protect humans against cancers of the colon, rectum, and thyroid.[6] Furthermore, the breakdown products of glucosinolates in the human body have been shown to exert a chemoprotective effect against dietary carcinogens in the alimentary canal through consumption studies in humans.[7][8] Saponins are also known as "soap-forming compounds" because they produce a soap-like foam when they occur in aqueous solutions. These compounds have been found to be antioxidants, antifungal and antiviral agents, although some of them induce jaundice and photosensitization,[9] and are used in detergents (soap-like), one example of which is the soapwort (Saponaria officinalis). Saponins can cause hemolysis and they have been used to develop bioactive hemolytic assays[10] and they have also been able to develop anti-adhesive activity compounds and strong hemolytic action.[11] Again, this compound also has an anti-cancer effect as seen in the reduction of types of B-cell lymphomas, as shown through a specific compound of ginseng (although it is not generalizing across all types of saponins).[12] Anthraquinone glycosides are a type of glycoside have a relatively limited distribution across the plant kingdom, however, they play a part in releasing electrolytes and peristalsis in the colon.[2] However, this has been associated with abuse and subsequent serious manifestations of diarrhea and severe hepatotoxicity and perhaps even renal failure.[13] They have also been shown to have potential toxicity and carcinogenicity.[14]
Flavonoids and Proanthrocyanidins
[edit]
These kinds of compounds are consistent with a three ring structure, as seen on the right, and proanthrocyanidins are oligomers of the flavonoids. They have activities as a general antioxidant, and studies have been carried out in many plants, and one example of this is of the Rosaceae family and its protection against the herpes simplex virus I (HSV-1), where it inhibited against the disease with not only a significant antioxidant activity but also a virucidal activity.[15] Flavonoids have also been shown to impact antioxidant power in black teas as well and were used to justify a clinical trial of their potential uses in medicine in Mauritius.[16]
Tannins
[edit]
There are two distinct types of tannins, condensed and hydrolysable tannins, which are polymers of flavonoids[2]. Usually, tannin has been used as the substances of vegetables which have been capable of transforming fresh hide into leather; however, in human health condensed tannins (hydrolysable tannins are more toxic) are used to bind indiscriminately to proteins.[2] Benefits have been assigned to some condensed tannins more than others, one example is the grape seed proanthrocyanidin extract (GSPE). Other plant examples which use the tannin (which is widely distributed throughout the plant kingdom) are Hamamelis virginiana (witch hazel) and Vaccinium macrocarpon (American Cranberry), which have been used to treat skin diseases and GI disorders, and protecting the urinary tract from UTIs respectively.[17]
Mono and Sequi-terpenoids, Phenylpropanoids, and Diterpenoids
[edit]
These compounds are made through the 5 carbon building block isoprene (diterpenoids are the exception) and they are, and they are very widely distributed throughout the plant kingdom and there are over 25,000 individual compounds identified.[2] In the Gingko plant, there is evidence that terpenoids are responsible for inhibiting the activity of the platelet activating factor, which is an important regulator of blood flow in the body. This can be used to treat memory loss, depression, or confusion, because it increases cerebral brain flow.[18] Another terpenoid is found in the licorice root (Glycyrrhiza glabra), called glycyrrhizin, which has been reported to induce interferon activity and augment natural killer cell activity. Also, it has been suggested that terpenoid possess activity to act against HIV, anti-inflammatory properties, and antiallergenic properties[18][19]
Resins are a compound that are part of terpenoids, however, they are a different type of a compound as they are complex lipid-soluble mixtures, and are sticky and the fluidity depends on the volatile compounds dissolved within them.[2] They work through regulating hydrogen and ammonium ions in the gut, and it depends how much of the resin is ingested (between 40-100g is healthy).[20] Possible complications of resin delivery is a potassium deficiency, or acidosis, however, in studies it has been shown to reduce LDL levels and increase HDL levels, which contributes to a decrease in coronary artery disease[21] Lignans are found in plant cell membranes and are generally lipophilic, and are found in oil seeds where they have been studied heavily. For example, they have been shown to be present in most fiber rich foods and has been shown to claim potential relief from the symptoms of menopause and lessen the risk of breast cancer. Furthermore, it has been shown that colon cancer can be reduced as a result of lignan consumption in the diet through suppression of lymphocyte proliferation[22]. Furthermore, it has been shown that the lignans consumed in lignan-rich foods are converted into a large part of the intestinal microflora in the upper part of the bowel to enterolactone and enterodiol, called mammalian or enterolignans. These breakdown products are important in the protective capabilities of lignan against chronic Western diseases. Flaxseed's benefit is primarily shown to be due to the antioxidant properties that it shows through lignan, due to the enterolactone and enterodiol products that were mentioned earlier and intestinal protection[23].
Alkaloids
[edit]
The alkaloids are nitrogen containing compounds which usually have a bitter taste.[2] They have been used in a multitude of clinical applications, including antimalarial, anticancer, Alzheimer's Disease, and antibiotic resistance.[24][25][26] Tropane alkaloids are present in Solanaceae (nightshade family), Datura (thorn apples), and Hyoscyamus niger (henbane) and are used to lessen smooth muscle spasms.[2] Scopolamine is the most important version of the tropane alkaloids, and it is found in various parts of the medical field: it is used in antiemesis, resuscitation, and serves as a compound used in the synthesis of next generation drugs. Furthermore, more notorious tropane alkaloids include cocaine, which is a derivative of ecgonine in which both tropane O— functional groups are esterified.[27] Isoquinoline compounds have an important range of activities, including myocardial contractility, as well as antinociceptive activity as well as exhibiting cytotoxicity in cancer cells.[28]
Furocoumarines and Naphthodianthrones
[edit]These compounds are important because they have a antidepressent effect and are used as a "folk remedy" to treat inflammation, as well as having antifungal properties[29]. These compounds, for the plant, protect it against herbivores. Plants which are able to synthesize these compounds which are used in medicine include Apiacae (carrot family), Fagopyrum esculeentum (buckwheat), and in Hypericum spp (St. John's-worts) of Clusiaceae (garcinia family)
See also[edit]
[edit]References
[edit]- ^ "Common Sense Medicine". wikipeidia.com. Retrieved 2018-02-03.
- ^ a b c d e f g h i j Bernhoft, Aksel (2010). "A brief review on bioactive compounds in plants". 11: 7 – via Google Scholar.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Glycoside". Wikipedia. 2018-03-19.
- ^ Newman, Robert A.; Yang, Peiying; Pawlus, Alison D.; Block, Keith I. (2008-02-01). "Cardiac Glycosides as Novel Cancer Therapeutic Agents". Molecular Interventions. 8 (1): 36. doi:10.1124/mi.8.1.8. ISSN 1534-0384. PMID 18332483.
- ^ Frese, Steffen; Frese-Schaper, Manuela; Andres, Anne-Catherine; Miescher, Daniela; Zumkehr, Beatrice; Schmid, Ralph A. (2006-06-01). "Cardiac glycosides initiate Apo2L/TRAIL-induced apoptosis in non-small cell lung cancer cells by up-regulation of death receptors 4 and 5". Cancer Research. 66 (11): 5867–5874. doi:10.1158/0008-5472.CAN-05-3544. ISSN 0008-5472. PMID 16740726.
- ^ a b Mithen, Richard F; Dekker, Matthijs; Verkerk, Ruud; Rabot, Sylvie; Johnson, Ian T (2000-05-15). "The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods". Journal of the Science of Food and Agriculture. 80 (7). doi:10.1002/(sici)1097-0010(20000515)80:7%3C967::aid-jsfa597%3E3.0.co;2-v. ISSN 1097-0010.
- ^ Shapiro, T. A.; Fahey, J. W.; Wade, K. L.; Stephenson, K. K.; Talalay, P. (1998-12-01). "Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables". Cancer Epidemiology and Prevention Biomarkers. 7 (12): 1091–1100. ISSN 1055-9965. PMID 9865427.
- ^ Verkerk, Ruud; Schreiner, Monika; Krumbein, Angelika; Ciska, Ewa; Holst, Birgit; Rowland, Ian; Schrijver, Remi De; Hansen, Magnor; Gerhäuser, Clarissa (2009-09-01). "Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health". Molecular Nutrition & Food Research. 53 (S2): S219–S219. doi:10.1002/mnfr.200800065. ISSN 1613-4133.
- ^ Francis, George; Kerem, Zohar; Makkar, Harinder P. S.; Becker, Klaus (2002/12). "The biological action of saponins in animal systems: a review". British Journal of Nutrition. 88 (6): 587–605. doi:10.1079/BJN2002725. ISSN 1475-2662.
{{cite journal}}: Check date values in:|date=(help) - ^ "Hemolysis of human erythrocytes with saponin affects the membrane structure". Acta Histochemica. 102 (1): 21–35. 2000-01-01. doi:10.1078/0065-1281-00534. ISSN 0065-1281.
- ^ "Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-κB activation in RAW 264.7 cells". Life Sciences. 76 (20): 2315–2328. 2005-04-01. doi:10.1016/j.lfs.2004.10.042. ISSN 0024-3205.
- ^ MOCHIZUKI, Mami; YOO, YungChoon; MATSUZAWA, Kaori; SATO, Katsuaki; SAIKI, Ikuo; TONOOKA, Shuichi; SAMUKAWA, Keiichi; AZUMA, Ichiro (1995-09-15). "Inhibitory Effect of Tumor Metastasis in Mice by Saponins, Ginsenoside-Rb2, 20(R)- and 20(S)-Ginsenoside-Rg3, of Red ginseng". Biological & Pharmaceutical Bulletin. 18 (9): 1197–1202. doi:10.1248/bpb.18.1197. ISSN 0918-6158.
- ^ Vanderperren, Bénédicte; Rizzo, Michela; Angenot, Luc; Haufroid, Vincent; Jadoul, Michel; Hantson, Philippe (July 2005). "Acute liver failure with renal impairment related to the abuse of senna anthraquinone glycosides". The Annals of Pharmacotherapy. 39 (7–8): 1353–1357. doi:10.1345/aph.1E670. ISSN 1060-0280. PMID 15956233.
- ^ "A review of the toxicity and carcinogenicity of anthraquinone derivatives". Toxicology. 57 (3): 227–240. 1989-08-01. doi:10.1016/0300-483X(89)90113-3. ISSN 0300-483X.
- ^ Shahat, Abdelaaty A.; Cos, Paul; De Bruyne, Tess; Apers, Sandra; Hammouda, Fayza M.; Ismail, Shams I.; Azzam, Safa; Claeys, Magda; Goovaerts, Etienne (June 2002). "Antiviral and antioxidant activity of flavonoids and proanthocyanidins from Crataegus sinaica". Planta Medica. 68 (6): 539–541. doi:10.1055/s-2002-32547. ISSN 0032-0943. PMID 12094299.
- ^ "Characterization of the antioxidant functions of flavonoids and proanthocyanidins in Mauritian black teas". Food Research International. 38 (4): 357–367. 2005-05-01. doi:10.1016/j.foodres.2004.10.005. ISSN 0963-9969.
- ^ Dixon, Richard A.; Xie, De-Yu; Sharma, Shashi B. (2005-01-01). "Proanthocyanidins - a final frontier in flavonoid research?". New Phytologist. 165 (1): 9–28. doi:10.1111/j.1469-8137.2004.01217.x. ISSN 1469-8137.
- ^ a b Craig, Winston J (1999-09-01). "Health-promoting properties of common herbs". The American Journal of Clinical Nutrition. 70 (3): 491s–499s. doi:10.1093/ajcn/70.3.491s. ISSN 0002-9165.
- ^ CRAIG, WINSTON J (1997-10-01). "Phytochemicals: Guardians of our Health". Journal of the American Dietetic Association. 97 (10). doi:10.1016/S0002-8223(97)00765-7. ISSN 0002-8223.
- ^ Djerassi, Carl; Geller, L. E.; Lemin, A. J. (1954-08-01). "Terpenoids. VIII.1 The Structures of the Cactus Triterpenes Gummosogenin and Longispinogenin2,3". Journal of the American Chemical Society. 76 (16): 4089–4091. doi:10.1021/ja01645a009. ISSN 0002-7863.
- ^ Brown, B.Greg; Zambon, Alberto; Poulin, Drew; Rocha, Anita; Maher, Vincent M.G; Davis, Joseph W; Albers, John J; Brunzell, John D (1998-02-26). "Use of Niacin, Statins, and Resins in Patients With Combined Hyperlipidemia". American Journal of Cardiology. 81 (4). doi:10.1016/S0002-9149(98)00039-3. ISSN 0002-9149.
- ^ Cassidy, Aedin; Hanley, Bryan; Lamuela‐Raventos, Rosa M (2000-05-15). "Isoflavones, lignans and stilbenes – origins, metabolism and potential importance to human health". Journal of the Science of Food and Agriculture. 80 (7). doi:10.1002/(sici)1097-0010(20000515)80:7%3C1044::aid-jsfa586%3E3.0.co;2-n. ISSN 1097-0010.
- ^ Touré, Alhassane; Xueming, Xu (2010-05-01). "Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits". Comprehensive Reviews in Food Science and Food Safety. 9 (3): 261–269. doi:10.1111/j.1541-4337.2009.00105.x. ISSN 1541-4337.
- ^ Ruchirawat, Prasat Kittakoop, Chulabhorn Mahidol and Somsak (2013-12-31). "Alkaloids as Important Scaffolds in Therapeutic Drugs for the Treatments of Cancer, Tuberculosis, and Smoking Cessation". Current Topics in Medicinal Chemistry. 14 (2).
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cesario, P. Russo, A. Frustaci, A. Del Bufalo, M. Fini and A. (2013-03-31). "Multitarget Drugs of Plants Origin Acting on Alzheimer's Disease". Current Medicinal Chemistry. 20 (13).
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cushnie, T.P. Tim; Cushnie, Benjamart; Lamb, Andrew J. (2014-11-05). "Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities". International Journal of Antimicrobial Agents. 44 (5): 377–386. doi:10.1016/j.ijantimicag.2014.06.001.
- ^ Grynkiewicz, Grzegorz; Gadzikowska, Maria (July 2008). "Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs". Pharmacological reports: PR. 60 (4): 439–463. ISSN 1734-1140. PMID 18799813.
- ^ "A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species". Life Sciences. 72 (6): 645–657. 2002-12-27. doi:10.1016/S0024-3205(02)02200-2. ISSN 0024-3205.
- ^ KOH; ONG (1999-04-01). "Phytophotodermatitis due to the application of Citrus hystrix as a folk remedy". British Journal of Dermatology. 140 (4): 737–738. doi:10.1046/j.1365-2133.1999.02782.x. ISSN 1365-2133.


