User:CBao2024/Alzheimer's Disease in the East Asian Population

Alzheimer's disease (AD) is a complex neurodegenerative disease that affects millions of people across the globe.[1] It is also a topic of interest in the East Asian population, especially as the burden of disease increases due to aging and population growth.[2] The pathogenesis of AD between ethnic groups is different.[3] However, prior studies in AD pathology have focused primarily on populations of European ancestry and may not give adequate insight on the genetic, clinical, and biological differences found in East Asians with AD.[4] Gaps in knowledge regarding Alzheimer's disease in the East Asian population introduce serious barriers to screening, early prevention, diagnosis, treatment, and timely intervention.[4]
Alzheimer's Disease
[edit]Alzheimer's disease is a progressive and irreversible brain disorder that impacts memory, cognitive function, personality, and behavior.[1] It is the cause of the majority of cases of dementia and involves increasing cognitive and functional impairment that interferes with a person's daily life. [1]

Symptoms
[edit]The early symptoms of Alzheimer's disease generally manifest as mild memory impairment and difficulty with everyday tasks.[5] As the disease progresses, affected individuals may experience disorientation, confusion, language problems, impaired judgment, and personality changes.[5] This disease is divided into mild, moderate, and severe stages.[5] During early stages, patients are capable of functioning normally but exhibit noticeable memory and cognitive issues.[5] During later stages, a patient's independence decreases as disease progresses and many patients end up needing increasing levels of care.[5]
Pathology
[edit]Alzheimer's disease is characterized by widespread neurodegeneration and the accumulation of abnormal protein deposits in the brain, including amyloid beta (Aβ) plaques and neurofibrillary tangles consisting of tau proteins.[1] These deposits impact the connectivity and function of neurons and can lead to cell death.[1] The pathogenesis of AD also involves aberrant immune regulation, inflammation, altered vascular regulation, and impaired cholesterol homeostasis.[3]
Prevalance
[edit]
The prevalence of Alzheimer's disease and other dementias is significant in East Asian countries compared to the global average, which has 667 cases per 100,000 population.[2] Japan has the highest prevalence of AD in the world at 3,079 cases per 100,000 population.[2] South Korea and China have 1119 and 924 cases per 100,000 population, respectively.[2] China has the highest number of people with dementia in the world at 13.1 million cases.[2] Women were found to have higher rates of AD, [6] [7] [8]potentially due to higher estrogen levels.[9][7] The prevalence is higher in older individuals and in rural areas.[8] In North America, the rates of Alzheimer's disease in Asian Americans were found to be similar to Non-Hispanic Whites and lower compared to Black Americans and Hispanic Americans.[7][10] Japanese Americans were found to have increased AD prevalence compared to other Asian American subgroups.[11] In terms of trends, Japan has experienced a rapid increase in prevalence rates relative to the global average, from 772 cases per 100,000 in 1990 to 3029 cases per 100,000 in 2019.[2] Japan is also expected to have the highest dementia-related death rates by 2040.[2]
Risk Factors and Co-morbidities in East Asians
[edit]
Age and genetics have consistently been the strongest predictors of dementia and AD.[12][3] However, lifestyle factors and other co-morbidities can play a significant role in contributing to AD susceptibility.[13]
Demographic Risk factors
[edit]Older age is a significant risk factor for dementia, with the rate of cognitive decline being tenfold greater in the last three years of life.[12] Income, occupation, and education are associated with the risk of developing AD in East Asian populations.[14] Higher education generally indicates lower risk of AD.[14] More physical or unsafe workplace conditions are associated with increased risk of AD. [15]
Behavioral and Physiological Risk factors
[edit]Smoking [16][2], low social activity [17], and low physical activity [18] are risk factors for AD in East Asian populations. Vascular conditions such as high blood pressure are also linked to dementia later in life.[19] A sedentary lifestyle or obesity are believed to contribute to dementia susceptibility.[2] Specifically, lower body mass index (BMI) may contribute to a lower likelihood of dementia.[20] A healthy diet high in fish, fruit, vegetables, and legumes but lower in meat and dairy is associated with decreased risk of AD.[21] Regular consumption of vegetable and fruit juices may decrease AD risk due to the intake of popyphenols.[22] Abnormal levels of insulin and impaired glucose tolerance is connected to increased dementia susceptibility in East Asians.[23][24] Poor early life environmental factors contribute to longer duration or more rapidly progressing AD later in life.[25] Daytime sleepiness and sleep apnea may have a relationship with later cognitive decline and dementia.[14] A study of Japanese Americans found that mid-life proteinuria and impaired renal function may be an independent predictor of late-life dementia.[26]
Comorbidities
[edit]Diabetes, hypertension, cardiovascular diseases, stroke, and cancer are comorbidities in East Asian people with AD. [2][27] In a review of AD patients in Taiwan, the co-prevalence of hypertension, diabetes, and stroke was reported to range from 30.2% to 60.7%, 15.1% to 24.2%, and 10.8 to 13.7% respectively.[27] A study of Korean AD patients identified elevated prevalence of hypertension (71.6% AD vs 55% control) and diabetes (44.9% AD vs 30.1% control) in AD patients compared to control patients.[28] Depressive symptoms and diabetes were also more increased in cognitively impaired Chinese Americans compared to White Americans.[29]
Genetic Risk factors
[edit]See "Genetics of Alzheimer's Disease in East Asians."
Genetics of Alzheimer's Disease in East Asians
[edit]Alzheimer's disease is influenced by a multitude of contributing genetic factors. The heritability of AD is estimated to range from 58-79%, making the elucidation of the genetic determinants of disease a crucial component to understanding the pathology of AD in East Asians.[4]
Genetic Studies
[edit]Genetic studies such as genome-wide association studies (GWAS) are used to scan the genomes of many individuals to identify genetic variations that may be associated with a certain disease or trait.[30] GWAS in Alzheimer's disease patients have been able to identify a number of risk variants and genetic factors that are linked to disease.[4] Prior genetic studies have been focused primarily on populations with European ancestry, which prevents a comprehensive understanding of AD in other ethnic groups.[4] It is important for GWAS to be conducted across diverse populations because the genetic differences between ethnic groups may influence the underlying biology of AD and identify unique genetic determinants.[4] Only seven GWAS have been conducted using East Asian populations to identify race-specific genetic factors and susceptibility loci associated with AD.[4] These GWAS focused on using cohorts from China, South Korea, and Japan.[4] Compared to GWAS in Caucasians, GWAS in East Asians are also much smaller and have revealed 26 AD-associated loci in total.[4] In comparison, a single recent GWAS in European populations involved over a million individuals and have identified 38 susceptibility loci.[31]
ApoE
[edit]
The genotype of apolipoprotein E (ApoE) is the strongest genetic risk factor for sporadic AD.[32] ApoE is a multifunctional protein that is involved in lipid transport and exists in several different forms or alleles.[33]
| Odds Ratio (OR) of 1 is normal risk. Higher OR indicates higher risk. | |||||
|---|---|---|---|---|---|
| Population | Two copies
of ε2 allele |
No copies
of ε4 allele |
One copy
of ε4 allele |
Two copies
of ε4 allele |
Reference |
| Caucasian | 0.9 | 1 | 2.7 | 12.5 | [34][35] |
| Japanese | 1.1 | 1 | 5.6 | 33.1 | [34] |
| African
American |
2.4 | 1 | 1.1 | 5.5 | [34] |
| Hispanic | 2.6 | 1 | 2.2 | 2.2 | [34] |
The most common APOE alleles are the ε2, ε3, and ε4 alleles, which were discovered to each be associated with varying levels of risk for AD (See Table 1).[33] The ε4 allele has been confirmed to be a major risk factor for AD whereas the ε2 allele has been shown to be protective.[33] However, the effects of ApoE genotype on AD risk are different across ethnic groups. Notably, the ε4 allele has a stronger effect in East Asian populations compared to other populations.[4] The regional prevalence estimates of ε4 allele carrier frequencies are lowest in Asia, compared to Northern Europe where the regional prevalence estimates are highest.[36] The ApoE ε4 allele was shown to lead to an increased rate of cognitive function decline in Han Chinese in Taiwan.[37]
| Variant ID | Amino Acid
Change |
Pathogenicity | Related Disease |
|---|---|---|---|
| rs121918392 | p.Glu21Lys | Pathogenic | Hyperlipoproteinemia, type III; atherosclerosis (APOE ε5) |
| rs587778876 | p.Leu122Met | Unknown | Major depressive disorder |
| rs121918397 | p.Arg163His | Pathogenic | Familial hyperlipoproteinemia, type III |
| rs121918397 | p.Arg163Pro | Pathogenic | Lipoprotein glomerulopathy |
| rs267606663 | p.Arg242Gln | Pathogenic | Familial hyperlipoproteinemia, type III |
| rs140808909 | p.Glu262Lys | Pathogenic | Hyperlipoproteinemia, type III; atherosclerosis (APOE ε7) |
| rs190853081 | p.Glu263Lys | Pathogenic | Hyperlipoproteinemia, type III; atherosclerosis (APOE ε7) |
Low frequency genetic variants linked to the pathophysiology of AD are important for elucidating disease etiology. Rare missense variants of ApoE may have differing prevalence in East Asians with AD. [4] Recent research has focused on the Christchurch (rs121918393) and Jacksonville (rs199768005) variants, which appear to have protective effects against AD in Caucasians.[38][39] However, these variants have not yet been identified in AD patients of Japanese ancestry, suggesting that these variants may be exclusive to Caucasians. [4] Other ApoE variants that are detected only in East Asians have also been identified but they have not yet been linked to AD (See Table 2).[4]
APP, PSEN1, and PSEN2
[edit]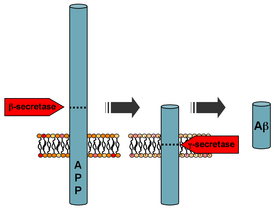
Mutations in APP (amyloid-beta precursor protein), PSEN1 (Presenilin-1), and PSEN2 (Presenilin-2) are known genetic causes of autosomal dominant forms of early-onset Alzheimer's disease (EOAD).[40] APP encodes the precursor protein to amyloid-beta.[40] Cleavage of APP by β-secretase and γ-secretase produces the pathogenic Aβ peptide in AD. PSEN1 and PSEN2 encode presenilin 1 and presenilin 2, which are major core components of the γ-secretase complex.[40] There are over 400 mutations in these genes reported worldwide and classification of variants in these genes across ethnic groups is difficult.[41] PSEN1 is the most frequently mutated and leads to the youngest age of of onset, followed by APP, and subsequently, PSEN2.[40] A small study of 200 early-onset AD patients in four East Asian countries found that 16% of patients presented pathogenic APP, PSEN1, or PSEN2 variants, 25% presented APP variants, 59% presented PSEN variants, and 16% presented PSEN2 variants.[40] Over 40 mutations in PSEN1/PSEN2/APP have been found in Han Chinese patients with EOAD, with 31 mutations in PSEN1, 4 mutations in PSEN2, and 5 mutations in APP.[41] The Chinese Familial AD Network, which enrolls 1330 patients in 404 pedigrees, found that 13.12% of pedigrees carry missense mutations in APP, PSEN1, and PSEN2.[42]
BIN1
[edit]Genetic variants of bridging integrator 1 (BIN1) gene have been shown to be significantly associated with Alzheimer’s disease in East Asians.[43] BIN1 is involved in endocytosis regulation and membrane remodeling, calcium homeostasis, DNA repair, cytoskeletal regulation, apoptosis, and inflammation.[44][45] Importantly, BIN1 has been identified as a critical genetic susceptibility loci for late-onset AD and is believed to modulate the tau pathology pathway.[44] Elevated BIN1 expression is associated with higher susceptibility for late-onset AD. [45]
Three SNP (single nucleotide polymorphisms) variants (rs12989701, rs744373, and rs7561528) in the BIN1 locus have been widely reported to be strongly associated with late-onset AD in Caucasian patients.[46] However, information on the risk profile of these SNPs in East Asians is missing or inconsistent across studies.[43] For instance, studies concerning the rs12989701 polymorphism have been primarily conducted in Caucasian patients, with limited data from the East Asian population.[47] Mutations in the other two SNPs, rs7561528 and rs744373, are reported to be generally associated with increased AD risk, although the results in East Asians are varied.[47] A few studies in East Asians have failed to reliably replicate a significant association between these polymorphisms and AD risk due to small sample size.[48] A 2012 study found no significant connection between rs7561528 or rs744373 and late-onset AD in patients of northern Han Chinese ancestry. [49] Similarly, 2015 study identified no association between rs7561528 and sporadic AD in Han Chinese populations [50] and a study in Japanese AD patients reported no significant association of rs744373 with late-onset AD.[51] Conversely, a 2015 study identified a significant link between rs7561528 and sporadic AD in the Han Chinese population.[52]
Meta-analysis of multiple independent studies provides a more comprehensive picture of the role of BIN1 polymorphisms in disease. A recent meta-analysis of 18 independent studies on rs7561528 in East Asians concluded that the A allele of this SNP is a protective factor against AD whereas the G allele confers elevated disease risk.[52] Similarly, a 2013 meta-analysis of pooled samples from two studies found that the rs744373 polymorphism had a similar genetic risk in Caucasian and East Asian populations.[48] Specifically, the AG and GG genotype of rs744373 is associated with increased AD risk.[53]
ABCA7
[edit]SNPs found in ABCA7, or ATP binding cassette subfamily A member 7, have been associated with increased early-onset and late-onset AD risk.[3] ABCA7 is an ATP-binding cassette (ABC) transporter protein that is important in the transport of lipids, cholesterol, apolipoprotein E (ApoE), and high density lipoprotein (HDL) across membranes.[3] ABCA7 plays a role in the regulation of amyloid-β homeostasis including amyloid clearance and tau fibril formation.[3] Functional interference of ABCA7 can lead to elevated amyloid-β production.[3] In the Southern Chinese population, polymorphisms rs3764650 and rs4147929 were reported to result in increased susceptibility for AD in ApoE ε4 carriers.[54] The rs3764650 variant is also associated with an earlier trajectory of cognitive decline in Han Chinese individuals in Taiwan.[37]
Other Genetic Loci associated with AD
[edit]Genetic studies have reported a connection between single nucleotide polymorphisms (SNPs) and AD risk.[3] The associated SNPs and genetic factors identified in East Asians are different compared to those found in other ethnic groups and can be an important early screening tool for AD in the East Asian population (See Table 3).[3] GWAS in Japanese cohorts conducted in 2013 and 2015 discovered disease-associated SNPs near SORL1, CNTNAP2, SUDS3, FAM47E, and SCARB2.[55][56][57] GWAS from Chinese cohorts identified SNPs in GCH1, APOC1, KCNJ15, LINC01413, RHOBTB3, GLRX, and other regions.[58][59] In South Korea, two recent GWAS of ApoE ε4 carriers identified novel AD-risk variants near SORCS1, CHD2, CACNA1A, and LRIG1.[60][61]
| Japanese Cohort | South Korean Cohort | Chinese Cohort | |||||
|---|---|---|---|---|---|---|---|
| Genetic Loci | Reference | Genetic Loci | Reference | Genetic Loci | Reference | Genetic Loci | Reference |
| SORL1 | [55][4] | CLIC4 | [60][4] | CHD2 | [60][4] | GCH1 | [58][4] |
| ENSG00000266602 | [56][4] | PTPRN2 | [60][4] | CACNA1A | [61][4] | LINC01413 | [58][4] |
| CNTNAP2 | [56][4] | PSD3 | [60][4] | LRIG1 | [61][4] | APOC1 | [58][4] |
| SUDS3 | [56][4] | SORCS1 | [60][4] | KCNJ15 | [58][4] | ||
| FAM47E | [57][4] | ENSG00000288047 | [60][4] | RHOBTB3 | [59][4] | ||
| SCARB2 | [57][4] | CHASERR | [60][4] | GLRX | [59][4] | ||
| ABR | [60][4] | ENSG00000252337 | [59][4] | ||||
| USP32 | [60][4] | LINC02325 | [59][4] | ||||
| SORCS1 | [60][4] | CHODL | [59][4] | ||||
Pathogenesis of AD in East Asians
[edit]Neurotransmitter Regulation
[edit]The regulation of monoaminergic and cholinergic neurotransmitters are relevant in Alzheimer's disease pathogenesis. Specifically, polymorphisms in GCH1 (GTP cyclohydrolase I) and ChAT (choline acetyltransferase) affect AD susceptibility in East Asians.[3] GTP cyclohydrolase I is an enzyme that synthesizes a protein tetrahydrobiopterin (THB) that necessary for monamine neurotransmitter synthesis.[62] The SNP rs72713460 of GCH1 impacts monoamine neurotransmitter synthesis and leads to increased AD susceptibility in East Asians.[58] Choline acetyltransferase (ChAT) is needed to generate acetylcholine from choline and acetyl COA.[63] Inhibition of acetylcholine (Ach) breakdown has been used to treat AD.[63] The rs3810950 polymorphism of ChAT was found to confer higher risk of AD in East Asians and may impact acetylcholine functionality. [64]
Amyloid-β Production and Clearance
[edit]The dynamics of amyloid-beta production and clearance are critically dysregulated in AD. Based on the amyloid cascade hypothesis, synthesized APP is transported to the cell surface and internalized by endocytosis for processing by α- or β- secretase, followed by γ-secretase.[65] β- and γ-secretases cleave APP into Aβ in the amyloidogenic pathway whereas α- and γ-secretase inhibit Aβ generation in the non-amyloidogenic pathway.[65] β-site APP-cleaving enzyme 1 (BACE1) is the β-secretase important for Aβ generation.[65]
| Gene | SNP | AD susceptibility
in East Asians |
Odds Ratio
Odds Ratio (OR) of 1 is normal risk. Higher OR indicates higher risk. |
|---|---|---|---|
| FEMRT2 | rs17125924 | Risk | 1.895 (East Asian) vs 1.14 (Caucasian) |
| GSK3β | rs334558 | Risk | 1.38 (East Asian) vs 1.99 (Caucasian) |
| CD2AP | rs934940 | Risk | 1.33 (East Asian) vs 1.11 (Caucasian) |
| BIN1 | rs7561528 | Risk | 1.02 (East Asian) vs 0.95 (Caucasian) |
| PICALM | rs3851179 | Protective | 0.88 (East Asian) vs 0.78 (Caucasian) |
| SORL1 | rs11218343 | Protective | 0.83 (East Asian) vs 0.75 (Caucasian) |
| rs3781834 | Protective | 0.74 (East Asian) vs 0.78 (Caucasian) | |
| rs4598682 | Protective | 0.75 (East Asian) vs 1.04 (Caucasian) | |
| rs17125523 | Protective | 0.77 (East Asian) vs 0.85 (Caucasian) | |
| rs3737529 | Protective | 0.77 (East Asian) vs 0.83 (Caucasian) | |
| SORCS1 | rs1890078 | Protective | 0.43 (East Asian) vs Unreported (Caucasian) |
| rs144835823 | Protective | 0.32 (East Asian) vs Unreported (Caucasian) | |
| rs78442236 | Protective | 0.17 (East Asian) vs Unreported (Caucasian) |
SNPs associated with AD risk have been identified on multiple genes involved in amyloid-β production and clearance (See Table 4). BIN1 and CD2AP work together in facilitating the endocytosis of APP and BACE1 into endosomes in order to produce amyloid-β.[3] The rs744373 SNP of BIN1 and rs934940 SNP of CD2AP confers increased AD susceptibility in East Asians.[3] FERMT2 (Kindlin-2) modulates APP metabolism and controls synaptic connectivity and axonal growth in a APP-dependent manner.[66] The rs7143400 SNP of FERMT2 is associated with a higher odds ratio of AD risk in East Asians compared to Caucasians.[3] Glycogen synthase kinase-3beta (GSK-3B) impacts the expression of BACE1, leading to effects on APP cleavage and amyloid-β deposition.[67] The rs334558 AD risk SNP of BACE1 has a higher odds ratio of risk in Caucasians relative to East Asians.[3] PICALM (phosphatidylinositol binding clathrin assembly protein) regulates the formation of vesicles in clathrin-mediated endocytosis.[68] Reduction of PICALM leads to decreased BACE1 activity, endocytosis, and Aβ production.[68] The rs3851179 polymorphism of PICALM, in association with the ε4 allele of ApoE, was significantly associated with a decreased risk of AD in a Korean population.[53]
Vascular Dysfunction
[edit]Vascular dysfunction is a contributor to Alzheimer's disease pathophysiology. Pathological pathways include blood brain barrier (BBB) dysfunction, disrupted clearance of amyloid beta, and altered neuromuscular coupling.[69] Multiple vascular processes are impacted by EXOC3L2 (Exocyst complex component 3-like 2) in East Asians with AD.[3] The rs597668 SNP on EXOC3L2 is a protective polymorphism in East Asians.[3] EXOC3L2 encodes a protein that is associated with an exocyst complex that regulates membrane dynamics.[70] EXOC3L2 influences vascular processes by decreasing vascular endothelial growth factor (VEGF).[3] Reduced VEGF leads to inhibition of angiogenesis and increased leucocyte adhesion, which results in obstructed cerebral blood flow.[3] Additionally, reduced VEGF increases breakdown of the blood brain barrier by amyloid-beta via inhibition of endothelial cells.[3] The resulting vascular dysfunction potentiates the deleterious effects of Alzheimer's disease.
Immune Regulation
[edit]The immune system plays an important role in AD pathogenesis.[71] Neuroinflammation has been found to be increased in the presence of elevated amyloid-beta deposition and abnormal tau aggregates.[71] Genetic mutations in immune-related genetic loci can also increase risk of AD via interference of normal immune function.[71] The immune system is believed undergo dynamic alterations and become dysregulated during disease progression.[71] In the East Asian population, multiple SNPs found in immune-related genes point to the role of immune system imbalance in AD (See Table 5).[3]
Certain SNPs have been correlated with increased susceptibility for AD in East Asians (See Table 5). TOMM40 encodes a translocase protein on the outer mitochondrial membrane that is involved in the import of Aβ into the mitochondria.[3] A number of SNPs on TOMM40 have been identified in East Asian AD patients and correlate with significantly increased susceptibility for disease. TOMM40 variants can lead to increased oxidative stress and mitochondrial function dysregulation. Similarly, Complement receptor type 1 (CR1) activation is deleterious to neurons because it inhibits microglia-mediated phagocytosis and stimulates reactive oxygen species (ROS) levels in the presence of amyloid-β.[3] The CR1 rs6656401 polymorphism has a slightly higher correlation with AD risk in Asians (odds ratio 1.76) compared to Caucasians (odds ratio 1.28).[3] KCNJ15 encodes the voltage-gated potassium channel Kir4.2 protein and is highly utilized in the immune system.[72] The rs928771 SNP of KCNJ15 leads to increased blood levels of KCNJ15 in AD patients as well as elevated disease risk in East Asians.[58] HLA-DRB1 (HLA class II histocompatibilty anteigen) is expressed on antigen-presenting cells and is involved in presenting peptides from extracellular proteins to T cells of the immune system.[73] The rs9271192 polymorphism of HLA-DRB1 is correlated with slightly increased risk in East Asians and Caucasians.[3] MTHFD1L (methylenetetrahydrofolate dehydrogenase (NADP + dependent) 1-like protein) is correlated with higher disease susceptibility but not much is known about what role it plays in AD.[3]
| Gene | SNP | AD susceptibility
in East Asians |
Odds Ratio
Odds Ratio (OR) of 1 is normal risk. Higher OR indicates higher risk. |
|---|---|---|---|
| TOMM40 | rs1155650 | Risk | 4.53 (East Asian) vs 3.13 (Caucasian) |
| rs157581 | Risk | 2.1 (East Asian) vs Unreported (Caucasian) | |
| MTHFD1L | rs11754661 | Risk | 1.83 (East Asian) vs 2.10 (Caucasian) |
| CR1 | rs6656401 | Risk | 1.76 (East Asian) vs 1.28 (Caucasian) |
| rs3818361 | Risk | 1.26 (East Asian) vs 1.26 (Caucasian) | |
| KCNJ15 | rs928771 | Risk | 1.59 (East Asian) vs Unreported (Caucasian) |
| HLA-DRB1 | rs9271192 | Risk | 1.07 (East Asian) vs 1.11 (Caucasian) |
| DAPK1 | rs4878104 | Protective | 0.75 (East Asian) vs 0.79 (Caucasian) |
| IL-18 | rs187238 | Protective | 0.669 (East Asian) vs 0.90 (Caucasian) |
| MS4A6A | rs610932 | Protective | 0.622 (East Asian) vs 0.91 (Caucasian) |
| CD33 | rs3865444 | Protective | 0.48 (East Asian) vs 1.1 (Caucasian) |
Protective polymorphisms in immune-related genes have also been identified in East Asians. DAPK1 (death-associated protein kinase) is a serine/threonine kinase that regulates various cellular pathways including apoptosis and autophagy.[74][3] The rs4878104 variant of DAPK has been found to be protective in both Asians and Caucasians.[3] Reduction of DAPK1 decreases caspase activation and was found to reduce memory deficits in mice injected with amyloid-β.[74] DAPK1 inhibition is also thought to reduce interleukin-18 (IL-18) production.[74] IL-18 is a proinflammatory cytokine that is linked to increased levels of amyloid-β accumulation though alterations of the APP processing.[3] The rs187238 variant of IL-18 is associated with more decreased risk of AD in East Asians, compared to Caucasians.[3] CD33 (sialic acid binding Ig-like lectin 3) is a transmembrane receptor protein that controls microglial activation.[3] In AD, it can be overactive in the presence of amyloid and contribute to excess neuroinflammation.[3] East Asians that carry a T allele of rs3865444 on the CD33 gene exhibit decreased levels of CD33 and may have decreased AD susceptibility.[75][3] Microglial inactivation due to CD33 inhibition results in decreased phagocytosis and cytokine release.[3] This SNP does not have a protective effect in Caucasians.[3] MS4A6A (Membrane Spanning 4-Domains A6A) is a member of the MS4A gene cluster and is important for immune cell activation.[76] The rs610932 risk variant of MS4A6A is associated with increased levels of MS4A6A expression in patients with mild cognitive impairment and AD.[76] High levels of MS4A6A expression is believed to have adverse effects in AD progression but limited research has been conducted on the exact role of MS4A6A in AD.[76][3]
Cultural and Societal Influences on AD treatment in East Asians
[edit]Attitudes relating to AD
[edit]Social stigma associated with mental illness and lack of knowledge about AD prevent proper management of disease in East Asians.[14] The misconception of AD as "insanity" is prevalent in East Asians as well as other ethnic groups.[77] Patients and caregivers may be reluctant to seek medical care due to this negative perception. One study of elderly Korean American immigrants found that there was limited utilization of mental health services despite elevated rates of cognitive impairment and depression this community. [78] Chinese patients were reported to seek care at later stages of dementia relative to American patients.[79] Memory loss, forgetfulness, and mental deterioration are also perceived to be a normal part of aging in East Asian American groups.[77] Multiple qualitative studies suggest this misconception is prevalent in Chinese, Vietnamese, and Korean Americans. [80][81][82] The cultural normalization of mental decline in old age may mask the initial symptoms of disease and delay formal evaluation in AD patients. [83][84] Feelings of shame linked to AD and limitations in AD-related knowledge are further accentuated in individuals with lower education levels and lower acculturation of AD.[85]
Barriers to Medical Care
[edit]A number of barriers result in disparities in adequate medical management and timely intervention for East Asians with AD or other dementias.[14] Delayed intervention due to insufficient healthcare, cultural influences, stigma against mental illness, and lack of AD knowledge inhibit proper management of disease.[14] Lack of accessible health care makes early recognition of AD symptoms challenging.[86][87] Additionally, healthcare systems and social services may not be able to meet the rising demand of AD treatment caused by aging and population growth.[2] Most significant is the growing burden of dementia in Japan, which is experiencing the fastest increase in AD prevalence in the world.[2] In China, the healthcare system is poorly prepared to deliver new disease-modifying AD treatments, with wait times predicted to be over two years.[88] The urban-rural divide in China is another infrastructure challenge for access to AD treatment.[88] In rural areas of China, local community centers or village doctors may be the primary point of contact for medical care, but may not have the formal training to assess cognitive impairment.[89]
Social and cultural factors may also be a barrier to medical care. The cultural norm of "saving face" in East Asians prevents public disclosure of negative family events, including AD. Lack of community discourse about AD may decrease awareness and knowledge about the early signs of disease.[90] Prior exposure to AD from multiple sources, such as via family members, media, or friends, is important in increasing disease awareness and AD knowledge.[91][92] Additionally, fear of social stigma makes it challenging for East Asian groups to seek proper medical services and poses a barrier for recruitment of East Asians in studies of mental illness. [83][84] Chinese AD patients were found to delay seeking care until they exhibited severe neuropsychiatric symptoms compared to Caucasian patients.[79] Elders in Asian American groups are not likely to be diagnosed with AD until later stages of disease.[93] These patients were more likely to be at a more severe stage of dementia by the time they sought medical attention, which poses consequences for treatment options.[79] Limited knowledge of AD results in delayed symptom recognition and poor disease management.[90] In Korean Americans and Chinese Americans, lower levels of formal education and acculturation were shown to be linked to lower levels of AD knowledge and decreased awareness of AD-related resources. [85][77][90] East Asians may interpret their mental health as a somatic issue and are less likely to utilize mental health services.[94] Some level of folk wisdom and skepticism of the availability of effective therapies are also factors that limit treatment-seeking behavior.[95]
Diagnosis
[edit]Fluid Biomarkers
[edit]The current research framework for diagnosis of Alzheimer's disease is based on the amyloid, tau, and and neurodegeneration (ATN) classification system.[96] This framework relies on cerebrospinal fluid sampling, MRI imaging, and PET imaging.[97] There is also interest in the use of blood-based biomarkers that may be more cost-effective and less invasive.[98] Plasma measurements of amyloid-β, neurofilament light chain (NfL), and phosphorylated tau (p-tau) can be used to diagnose or predict the development of AD.[99] Unfortunately, the studies highlighting the relationship between these biomarkers and future AD were performed primarily in Western patients.[99] Recent research has focused on the diagnostic and predictive capacity of fluid biomarkers in the East Asian population.[99] A study conducted in a Chinese population has shown that the combination of p-tau 181 and Aβ42 in plasma had excellent performance in diagnosing AD.[100] Several studies since have determined that combinations of plasma biomarkers, including NfL, Aβ42, and p-tau in plasma, can predict AD prognosis 4.8-6 years prior to cognitive decline. [101][102] Assessment of a combination of biomarkers has more predictive value than using just one biomarker.[99] These studies show that the relationship between plasma biomarkers and AD is preserved in East Asian populations and similar to findings in European populations.[99]
Imaging
[edit]PET and MRI imaging are important AD diagnostic tools but East Asians and other ethnic groups are underrepresented in studies on imaging-based AD biomarkers. One large multisite study of patients with mild cognitive impairment and dementia found that amyloid PET positivity rates were 7-12% lower in Asian individuals compared to White individuals.[103] In China, amyloid PET is rarely performed due to costs, coverage, and availability.[104] However, China is rapidly expanding its use of PET scanners. In 2023, Neuraceq (florbetaben F-18) was approved as the first PET imaging radiotracer targeting β-amyloid in China in response to recent approvals of promising AD treatments in the United States.[105] Neuraceq obtained approval in the U.S. in 2014, and is one of three PET radiopharmacheuticals approved by the FDA.[103]
References
[edit]- ^ a b c d e Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, et al. (May 2021). "Alzheimer disease". Nature Reviews Disease Primers. 7 (1): 33. doi:10.1038/s41572-021-00269-y. PMC 8574196. PMID 33986301
- ^ a b c d e f g h i j k l m Javaid, Syed Fahad; Giebel, Clarissa; Khan, Moien AB; Hashim, Muhammad Jawad (2021-05-27). Epidemiology of Alzheimer’s disease and other dementias: rising global burden and forecasted trends (Report). F1000Research.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al Besin, Valentinus; Humardani, Farizky Martriano; Mulyanata, Lisa Thalia (2023-06-01). "Neurogenomics of Alzheimer's disease (AD): An Asian population review". Clinica Chimica Acta; International Journal of Clinical Chemistry. 546: 117389. doi:10.1016/j.cca.2023.117389. ISSN 1873-3492. PMID 37211175.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap Miyashita, A., Kikuchi, M., Hara, N., & Ikeuchi, T. (2023). Genetics of Alzheimer's disease: an East Asian perspective. Journal of human genetics, 68(3), 115–124. https://doi.org/10.1038/s10038-022-01050-z
- ^ a b c d e Tahami Monfared, Amir Abbas; Byrnes, Michael J.; White, Leigh Ann; Zhang, Quanwu (2022-03-14). "Alzheimer's Disease: Epidemiology and Clinical Progression". Neurology and Therapy. 11 (2): 553–569. doi:10.1007/s40120-022-00338-8. ISSN 2193-8253. PMC 9095793. PMID 35286590.
- ^ Chang MH, Moonesinghe R, Athar HM, Truman BI (2016) Trends in Disparity by Sex and Race/Ethnicity for the Leading Causes of Death in the United States-1999-2010. Journal of public health management and practice : JPHMP 22 Suppl 1, S13–24
- ^ a b c Lim, S., Mohaimin, S., Min, D., Roberts, T., Sohn, Y. J., Wong, J., Sivanesathurai, R., Kwon, S. C., & Trinh-Shevrin, C. (2020). Alzheimer's Disease and its Related Dementias among Asian Americans, Native Hawaiians, and Pacific Islanders: A Scoping Review. Journal of Alzheimer's disease : JAD, 77(2), 523–537. https://doi.org/10.3233/JAD-200509
- ^ a b LI, Kanglan; WEI, Shouchao; LIU, Zhou; HU, Li; LIN, Jiajing; TAN, Shiting; MAI, Yingren; PENG, Wanjuan; MAI, Hui; HOU, Qi; TU, Guifeng (2018-11). "The Prevalence of Alzheimer's Disease in China: A Systematic Review and Meta-analysis". Iranian Journal of Public Health. 47 (11): 1615–1626. ISSN 2251-6085. PMC 6294855. PMID 30581776.
{{cite journal}}: Check date values in:|date=(help) - ^ Espeland MAB RD; Hugenschmidt C; Manson JE; Craft S; Yaffe K; Weitlauf J; Vaughan L; Johnson KC; Padula CB; Jackson RD; Resnick SM (2015) Impact of Type 2 Diabetes and Postmenopausal Hormone Therapy on Incidence of Cognitive Impairment in Older Women. Diabetes Care 38, 2316–2324.
- ^ Mayeda, Elizabeth Rose; Glymour, M.Maria; Quesenberry, Charles P.; Whitmer, Rachel A. (2016-02-10). "Inequalities in dementia incidence between six racial and ethnic groups over 14 years". Alzheimer's & Dementia. 12 (3): 216–224. doi:10.1016/j.jalz.2015.12.007. ISSN 1552-5260.
{{cite journal}}: no-break space character in|title=at position 80 (help) - ^ Hastings KG, Jose PO, Kapphahn KI, Frank ATH, Goldstein BA, Thompson CA, Eggleston K, Cullen MR, Palaniappan LP (2015) Leading causes of death among Asian American subgroups (2003-2011). PLoS ONE 10 (4) (no pagination)
- ^ a b Jansen, Willemijn J.; Wilson, Robert S.; Visser, Pieter Jelle; Nag, Sukriti; Schneider, Julie A.; James, Bryan D.; Leurgans, Sue E.; Capuano, Ana W.; Bennett, David A.; Boyle, Patricia A. (2018-01). "Age and the association of dementia-related pathology with trajectories of cognitive decline". Neurobiology of Aging. 61: 138–145. doi:10.1016/j.neurobiolaging.2017.08.029. ISSN 1558-1497. PMC 5721665. PMID 29078129.
{{cite journal}}: Check date values in:|date=(help) - ^ Popa-Wagner, Aurel; Dumitrascu, Dinu Iuliu; Capitanescu, Bogdan; Petcu, Eugen Bogdan; Surugiu, Roxana; Fang, Wen-Hui; Dumbrava, Danut-Adrian (2020-03). "Dietary habits, lifestyle factors and neurodegenerative diseases". Neural Regeneration Research. 15 (3): 394. doi:10.4103/1673-5374.266045. ISSN 1673-5374.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ a b c d e f Lim, Sahnah; Mohaimin, Sadia; Min, Deborah; Roberts, Timothy; Sohn, Young-Jin; Wong, Jazmine; Sivanesathurai, Ragavan; Kwon, Simona C.; Trinh-Shevrin, Chau (2020). "Alzheimer's Disease and its Related Dementias among Asian Americans, Native Hawaiians, and Pacific Islanders: A Scoping Review". Journal of Alzheimer's disease : JAD. 77 (2): 523–537. doi:10.3233/JAD-200509. ISSN 1387-2877. PMC 8638681. PMID 32675416.
- ^ Lim, S., Mohaimin, S., Min, D., Roberts, T., Sohn, Y. J., Wong, J., Sivanesathurai, R., Kwon, S. C., & Trinh-Shevrin, C. (2020). Alzheimer's Disease and its Related Dementias among Asian Americans, Native Hawaiians, and Pacific Islanders: A Scoping Review. Journal of Alzheimer's disease : JAD, 77(2), 523–537. https://doi.org/10.3233/JAD-200509
- ^ Tyas SL, White LR, Petrovitch H, Webster Ross G, Foley DJ, Heimovitz HK, Launer LJ (2003) Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiology of aging 24, 589–596.
- ^ Sacznski JS, Pfeifer LA, Masaki K, Korf ESC, Laurin D, White L, Launer LJ (2007) The effect of social engagement on incident dementia: The Honolulu-Asia aging study. Research and Practice in Alzheimer’s Disease 12, 42–48.
- ^ Smith TL, Masaki KH, Fong K, Abbott RD, Ross GW, Petrovitch H, Blanchette PL, White LR (2010) Effect of walking distance on 8-year incident depressive symptoms in elderly men with and without chronic disease: the Honolulu-Asia Aging Study. Journal of the American Geriatrics Society 58, 1447–1452.
- ^ Mayeda, Elizabeth Rose; Glymour, M Maria; Quesenberry, Charles P; Whitmer, Rachel A (2016-3). "Inequalities in dementia incidence between six racial and ethnic groups over 14 years". Alzheimer's & dementia : the journal of the Alzheimer's Association. 12 (3): 216–224. doi:10.1016/j.jalz.2015.12.007. ISSN 1552-5260. PMC 4969071. PMID 26874595.
{{cite journal}}: Check date values in:|date=(help) - ^ Gelber, Rebecca P.; Petrovitch, Helen; Masaki, Kamal H.; Abbott, Robert D.; Ross, G. Webster; Launer, Lenore J.; White, Lon R. (2012-1). "Lifestyle and the Risk of Dementia Among Japanese American Men". Journal of the American Geriatrics Society. 60 (1): 118–123. doi:10.1111/j.1532-5415.2011.03768.x. ISSN 0002-8614. PMC 3258374. PMID 22211390.
{{cite journal}}: Check date values in:|date=(help) - ^ Gelber, Rebecca P.; Petrovitch, Helen; Masaki, Kamal H.; Abbott, Robert D.; Ross, G. Webster; Launer, Lenore J.; White, Lon R. (2012-1). "Lifestyle and the Risk of Dementia Among Japanese American Men". Journal of the American Geriatrics Society. 60 (1): 118–123. doi:10.1111/j.1532-5415.2011.03768.x. ISSN 0002-8614. PMC 3258374. PMID 22211390.
{{cite journal}}: Check date values in:|date=(help) - ^ Dai Q, Borenstein AR, Wu Y, Jackson JC, Larson EB (2006) Fruit and vegetable juices and Alzheimer’s disease: the Kame Project. The American journal of medicine 119, 751–759.
- ^ Peila, Rita; Rodriguez, Beatriz L.; White, Lon R.; Launer, Lenore J. (2004-07-27). "Fasting insulin and incident dementia in an elderly population of Japanese-American men". Neurology. 63 (2): 228–233. doi:10.1212/01.wnl.0000129989.28404.9b. ISSN 1526-632X. PMID 15277613.
- ^ Curb, J. D.; Rodriguez, B. L.; Abbott, R. D.; Petrovitch, H.; Ross, G. W.; Masaki, K. H.; Foley, D.; Blanchette, P. L.; Harris, T.; Chen, R.; White, L. R. (1999-03-23). "Longitudinal association of vascular and Alzheimer's dementias, diabetes, and glucose tolerance". Neurology. 52 (5): 971–975. doi:10.1212/wnl.52.5.971. ISSN 0028-3878. PMID 10102414.
- ^ Graves, A. B.; Mortimer, J. A.; Larson, E. B.; Wenzlow, A.; Bowen, J. D.; McCormick, W. C. (1996-07). "Head circumference as a measure of cognitive reserve. Association with severity of impairment in Alzheimer's disease". The British Journal of Psychiatry: The Journal of Mental Science. 169 (1): 86–92. doi:10.1192/bjp.169.1.86. ISSN 0007-1250. PMID 8818374.
{{cite journal}}: Check date values in:|date=(help) - ^ Higuchi, Masaya; Chen, Randi; Abbott, Robert D.; Bell, Christina; Launer, Lenore; Ross, G. Webster; Petrovitch, Helen; Masaki, Kamal (2015-07). "Mid-Life Proteinuria and Late-Life Cognitive Function and Dementia in Elderly Men: The Honolulu-Asia Aging Study". Alzheimer disease and associated disorders. 29 (3): 200. doi:10.1097/WAD.0000000000000082. PMID 25626635.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Lanctôt, Krista L.; Hahn-Pedersen, J. Hviid; Eichinger, C. S.; Freeman, C.; Clark, A.; Tarazona, L. R. S.; Cummings, J. (2023-06-01). "Burden of Illness in People with Alzheimer's Disease: A Systematic Review of Epidemiology, Comorbidities and Mortality". The Journal of Prevention of Alzheimer's Disease: 1–11. doi:10.14283/jpad.2023.61. ISSN 2426-0266.
- ^ Lyou, Hyun Ji; Seo, Kwon-Duk; Lee, Ji Eun; Pak, Hae Yong; Lee, Jun Hong (2018-12-01). "Association of Alzheimer's Disease with the Risk of Developing Epilepsy: a 10-Year Nationwide Cohort Study". Dementia and Neurocognitive Disorders. 17 (4): 156–162. doi:10.12779/dnd.2018.17.4.156. ISSN 1738-1495. PMC 6425886. PMID 30906405.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Chao, Steven Z.; Matthews, Brandy R.; Yokoyama, Jennifer S.; Betty Lai, Ngan; Ong, Hilary; Tse, Marian; Yuan, Runfen Frances; Lin, Amy; Kramer, Joel; Yaffe, Kristine; Miller, Bruce L.; Rosen, Howard J. (2014-7). "Depressive symptoms in Chinese Americans with Cognitive Impairment". The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 22 (7): 642–652. doi:10.1016/j.jagp.2012.10.029. ISSN 1064-7481. PMC 4309267. PMID 24021225.
{{cite journal}}: Check date values in:|date=(help) - ^ Uffelmann, Emil; Huang, Qin Qin; Munung, Nchangwi Syntia; de Vries, Jantina; Okada, Yukinori; Martin, Alicia R.; Martin, Hilary C.; Lappalainen, Tuuli; Posthuma, Danielle (2021-08-26). "Genome-wide association studies". Nature Reviews Methods Primers. 1 (1): 1–21. doi:10.1038/s43586-021-00056-9. ISSN 2662-8449.
- ^ Wightman, Douglas P.; Jansen, Iris E.; Savage, Jeanne E.; Shadrin, Alexey A.; Bahrami, Shahram; Holland, Dominic; Rongve, Arvid; Børte, Sigrid; Winsvold, Bendik S.; Drange, Ole Kristian; Martinsen, Amy E.; Skogholt, Anne Heidi; Willer, Cristen; Bråthen, Geir; Bosnes, Ingunn (2021-09). "A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer's disease". Nature Genetics. 53 (9): 1276–1282. doi:10.1038/s41588-021-00921-z. ISSN 1546-1718. PMID 34493870.
{{cite journal}}: Check date values in:|date=(help) - ^ Serrano-Pozo, Alberto; Das, Sudeshna; Hyman, Bradley T. (2021-01). "APOE and Alzheimer's disease: advances in genetics, pathophysiology, and therapeutic approaches". The Lancet. Neurology. 20 (1): 68–80. doi:10.1016/S1474-4422(20)30412-9. ISSN 1474-4465. PMC 8096522. PMID 33340485.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Husain, Mohammed Amir; Laurent, Benoit; Plourde, Mélanie (2021). "APOE and Alzheimer's Disease: From Lipid Transport to Physiopathology and Therapeutics". Frontiers in Neuroscience. 15. doi:10.3389/fnins.2021.630502/full. ISSN 1662-453X.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d Farrer, L. A. (1997-10-22). "Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium". JAMA: The Journal of the American Medical Association. 278 (16): 1349–1356. doi:10.1001/jama.278.16.1349. ISSN 0098-7484.
- ^ Bertram, Lars; McQueen, Matthew B; Mullin, Kristina; Blacker, Deborah; Tanzi, Rudolph E (2007-01). "Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database". Nature Genetics. 39 (1): 17–23. doi:10.1038/ng1934. ISSN 1061-4036.
{{cite journal}}: Check date values in:|date=(help) - ^ Ward, Alex; Crean, Sheila; Mercaldi, Catherine J.; Collins, Jenna M.; Boyd, Dylan; Cook, Michael N.; Arrighi, H. Michael (2012). "Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer's disease: a systematic review and meta-analysis". Neuroepidemiology. 38 (1): 1–17. doi:10.1159/000334607. ISSN 1423-0208. PMID 22179327.
- ^ a b Hsieh, Tsung-Jen; Lee, Wei-Ju; Liao, Yi-Chu; Hsu, Chih-Cheng; Fang, Yao-Hwei; Chen, Tzu-Yu; Lin, Yung-Shuan; Chang, I.-Shou; Wang, Shuu-Jiun; Hsiung, Chao A.; Fuh, Jong-Ling; Alzheimer’s Disease Neuroimaging Initiative (2021-07-02). "Association between Alzheimer's disease genes and trajectories of cognitive function decline in Han Chinese in Taiwan". Aging. 13 (13): 17237–17252. doi:10.18632/aging.203204. ISSN 1945-4589. PMC 8312434. PMID 34214049.
- ^ Zalocusky, Kelly A.; Nelson, Maxine R.; Huang, Yadong (2019-11). "An Alzheimer's-disease-protective APOE mutation". Nature Medicine. 25 (11): 1648–1649. doi:10.1038/s41591-019-0634-9. ISSN 1546-170X.
{{cite journal}}: Check date values in:|date=(help) - ^ Medway, Christopher W.; Abdul-Hay, Samer; Mims, Tynickwa; Ma, Li; Bisceglio, Gina; Zou, Fanggeng; Pankratz, Shane; Sando, Sigrid B.; Aasly, Jan O.; Barcikowska, Maria; Siuda, Joanna; Wszolek, Zbigniew K.; Ross, Owen A.; Carrasquillo, Minerva; Dickson, Dennis W. (2014-03-10). "ApoE variant p.V236E is associated with markedly reduced risk of Alzheimer's disease". Molecular Neurodegeneration. 9 (1): 11. doi:10.1186/1750-1326-9-11. ISSN 1750-1326. PMC 3995879. PMID 24607147.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b c d e Giau, Vo Van; Bagyinszky, Eva; Youn, Young Chul; An, Seong Soo A.; Kim, SangYun (2019-09-25). "APP, PSEN1, and PSEN2 Mutations in Asian Patients with Early-Onset Alzheimer Disease". International Journal of Molecular Sciences. 20 (19): 4757. doi:10.3390/ijms20194757. ISSN 1422-0067. PMC 6801447. PMID 31557888.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Lin, Yung-Shuan; Cheng, Chih-Ya; Liao, Yi-Chu; Hong, Chen-Jee; Fuh, Jong-Ling (2020-11-13). "Mutational analysis in familial Alzheimer's disease of Han Chinese in Taiwan with a predominant mutation PSEN1 p.Met146Ile". Scientific Reports. 10 (1): 19769. doi:10.1038/s41598-020-76794-9. ISSN 2045-2322.
- ^ Jia, Longfei; Fu, Yue; Shen, Luxi; Zhang, Heng; Zhu, Min; Qiu, Qiongqiong; Wang, Qi; Yan, Xin; Kong, Chaojun; Hao, Jing; Wei, Cuibai; Tang, Yi; Qin, Wei; Li, Ying; Wang, Fen (2020-01). "PSEN1, PSEN2 , and APP mutations in 404 Chinese pedigrees with familial Alzheimer's disease". Alzheimer's & Dementia. 16 (1): 178–191. doi:10.1002/alz.12005. ISSN 1552-5260.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Zhou, Futao; Haina, Dong (2017-06-01). "The bridging integrator 1 Gene rs7561528 polymorphism contributes to Alzheimer's disease susceptibility in East Asian and Caucasian populations". Clinica Chimica Acta. 469: 13–21. doi:10.1016/j.cca.2017.03.013. ISSN 0009-8981.
- ^ a b Tan, Meng-Shan; Yu, Jin-Tai; Tan, Lan (2013-10). "Bridging integrator 1 (BIN1): form, function, and Alzheimer's disease". Trends in Molecular Medicine. 19 (10): 594–603. doi:10.1016/j.molmed.2013.06.004. ISSN 1471-499X. PMID 23871436.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Prokic, Ivana; Cowling, Belinda S.; Laporte, Jocelyn (2014-05-01). "Amphiphysin 2 (BIN1) in physiology and diseases". Journal of Molecular Medicine. 92 (5): 453–463. doi:10.1007/s00109-014-1138-1. ISSN 1432-1440.
- ^ Hu, Xiaolan; Pickering, Eve; Liu, Yingxue Cathy; Hall, Stephanie; Fournier, Helene; Katz, Elyse; Dechairo, Bryan; John, Sally; Eerdewegh, Paul Van; Soares, Holly; Initiative, the Alzheimer's Disease Neuroimaging (2011-02-24). "Meta-Analysis for Genome-Wide Association Study Identifies Multiple Variants at the BIN1 Locus Associated with Late-Onset Alzheimer's Disease". PLOS ONE. 6 (2): e16616. doi:10.1371/journal.pone.0016616. ISSN 1932-6203. PMC 3044719. PMID 21390209.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b Dong, Xiaoliu; Zhang, Li; Meng, Qingling; Gao, Qiuyan (2017-01-01). "Association Between Interleukin-1A, Interleukin-1B, and Bridging integrator 1 Polymorphisms and Alzheimer's Disease: a standard and Cumulative Meta-analysis". Molecular Neurobiology. 54 (1): 736–747. doi:10.1007/s12035-015-9683-3. ISSN 1559-1182.
- ^ a b Liu, Guiyou; Zhang, Shuyan; Cai, Zhiyou; Li, You; Cui, Lili; Ma, Guoda; Jiang, Yongshuai; Zhang, Liangcai; Feng, Rennan; Liao, Mingzhi; Chen, Zugen; Zhao, Bin; Li, Keshen (2013-06-07). "BIN1 gene rs744373 polymorphism contributes to Alzheimer's disease in East Asian population". Neuroscience Letters. 544: 47–51. doi:10.1016/j.neulet.2013.02.075. ISSN 0304-3940.
- ^ Tan, Lan; Yu, Jin-Tai; Zhang, Wei; Wu, Zhong-Chen; Zhang, Qun; Liu, Qiu-Yan; Wang, Wei; Wang, Hui-Fu; Ma, Xiao-Ying; Cui, Wei-Zhen (2013-09-01). "Association of GWAS-linked loci with late-onset Alzheimer's disease in a northern Han Chinese population". Alzheimer's & Dementia. 9 (5): 546–553. doi:10.1016/j.jalz.2012.08.007. ISSN 1552-5260.
{{cite journal}}: no-break space character in|title=at position 71 (help) - ^ Xiao, Qianyi; Liu, Zhi-Jun; Tao, Sha; Sun, Yi-Min; Jiang, Deke; Li, Hong-Lei; Chen, Haitao; Liu, Xu; Lapin, Brittany; Wang, Chi-Hsiung; Zheng, S. Lilly; Xu, Jianfeng; Wu, Zhi-Ying (2015-11-10). "Risk prediction for sporadic Alzheimer's disease using genetic risk score in the Han Chinese population". Oncotarget. 6 (35): 36955–36964. doi:10.18632/oncotarget.6271. ISSN 1949-2553. PMC 4741908. PMID 26543236.
- ^ Ohara, Tomoyuki; Ninomiya, Toshiharu; Hirakawa, Yoichiro; Ashikawa, Kyota; Monji, Akira; Kiyohara, Yutaka; Kanba, Shigenobu; Kubo, Michiaki (2012-12). "Association study of susceptibility genes for late-onset Alzheimer's disease in the Japanese population". Psychiatric Genetics. 22 (6): 290–293. doi:10.1097/YPG.0b013e3283586215. ISSN 1473-5873. PMID 22935915.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Li, Hong-Lei; Yang, Ping; Liu, Zhi-Jun; Sun, Yi-Min; Lu, Shen-Ji; Tao, Qing-Qing; Guo, Qi-Hao; Wu, Zhi-Ying (2015-02). "Common variants at Bin1 are associated with sporadic Alzheimer's disease in the Han Chinese population". Psychiatric Genetics. 25 (1): 21–25. doi:10.1097/YPG.0000000000000071. ISSN 1473-5873. PMID 25461955.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Kim, Sanghee; Mun, Myung-Jin; Jin-ho; Kim; Jang, W. (2016). "Associations of Three Polymorphisms in Endocytosis-related Genes with the Risk of Alzheimer ' s Disease in Korean and East Asian Populations".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Wang, Lijun; Jiao, Yang; Zhao, Aonan; Xu, Xiaomeng; Ye, Guanyu; Zhang, Yichi; Wang, Ying; Deng, Yulei; Xu, Wei; Liu, Jun (2022). "Analysis of Genetic Association Between ABCA7 Polymorphism and Alzheimer's Disease Risk in the Southern Chinese Population". Frontiers in Aging Neuroscience. 14. doi:10.3389/fnagi.2022.819499/full. ISSN 1663-4365.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Miyashita, Akinori; Koike, Asako; Jun, Gyungah; Wang, Li-San; Takahashi, Satoshi; Matsubara, Etsuro; Kawarabayashi, Takeshi; Shoji, Mikio; Tomita, Naoki; Arai, Hiroyuki; Asada, Takashi; Harigaya, Yasuo; Ikeda, Masaki; Amari, Masakuni; Hanyu, Haruo (2013). "SORL1 is genetically associated with late-onset Alzheimer's disease in Japanese, Koreans and Caucasians". PloS One. 8 (4): e58618. doi:10.1371/journal.pone.0058618. ISSN 1932-6203. PMC 3614978. PMID 23565137.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d Hirano, Atsushi; Ohara, Tomoyuki; Takahashi, Atsushi; Aoki, Masayuki; Fuyuno, Yuta; Ashikawa, Kyota; Morihara, Takashi; Takeda, Masatoshi; Kamino, Kouzin; Oshima, Etsuko; Okahisa, Yuko; Shibata, Nobuto; Arai, Heii; Akatsu, Hiroyasu; Ikeda, Masashi (2015-08). "A genome-wide association study of late-onset Alzheimer's disease in a Japanese population". Psychiatric Genetics. 25 (4): 139–146. doi:10.1097/YPG.0000000000000090. ISSN 1473-5873. PMID 26049409.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Shigemizu, Daichi; Mitsumori, Risa; Akiyama, Shintaro; Miyashita, Akinori; Morizono, Takashi; Higaki, Sayuri; Asanomi, Yuya; Hara, Norikazu; Tamiya, Gen; Kinoshita, Kengo; Ikeuchi, Takeshi; Niida, Shumpei; Ozaki, Kouichi (2021-03-03). "Ethnic and trans-ethnic genome-wide association studies identify new loci influencing Japanese Alzheimer's disease risk". Translational Psychiatry. 11 (1): 151. doi:10.1038/s41398-021-01272-3. ISSN 2158-3188. PMC 7925686. PMID 33654092.
- ^ a b c d e f g Zhou, Xiaopu; Chen, Yu; Mok, Kin Y.; Zhao, Qianhua; Chen, Keliang; Chen, Yuewen; Hardy, John; Li, Yun; Fu, Amy K. Y.; Guo, Qihao; Ip, Nancy Y.; Alzheimer’s Disease Neuroimaging Initiative (2018-02-20). "Identification of genetic risk factors in the Chinese population implicates a role of immune system in Alzheimer's disease pathogenesis". Proceedings of the National Academy of Sciences of the United States of America. 115 (8): 1697–1706. doi:10.1073/pnas.1715554115. ISSN 1091-6490. PMC 5828602. PMID 29432188.
- ^ a b c d e f Jia, Longfei; Li, Fangyu; Wei, Cuibai; Zhu, Min; Qu, Qiumin; Qin, Wei; Tang, Yi; Shen, Luxi; Wang, Yanjiang; Shen, Lu; Li, Honglei; Peng, Dantao; Tan, Lan; Luo, Benyan; Guo, Qihao (2021-04-12). "Prediction of Alzheimer's disease using multi-variants from a Chinese genome-wide association study". Brain: A Journal of Neurology. 144 (3): 924–937. doi:10.1093/brain/awaa364. ISSN 1460-2156. PMC 8041344. PMID 33188687.
- ^ a b c d e f g h i j k Park, Jong Ho; Park, Inho; Youm, Emilia Moonkyung; Lee, Sejoon; Park, June Hee; Lee, Jongan; Lee, Dong Young; Byun, Min Soo; Lee, Jun Ho; Yi, Dahyun; Chung, Sun Ju; Park, Kye Won; Choi, Nari; Kim, Seong Yoon; Yoon, Woon (2021-06). "Novel Alzheimer's disease risk variants identified based on whole-genome sequencing of APOE ε4 carriers". Translational Psychiatry. 11 (1). doi:10.1038/s41398-021-01412-9. ISSN 2158-3188.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Kang, Sarang; Gim, Jungsoo; Lee, Jiwoon; Gunasekaran, Tamil Iniyan; Choi, Kyu Yeong; Lee, Jang Jae; Seo, Eun Hyun; Ko, Pan Woo; Chung, Ji Yeon; Choi, Seong Min; Lee, Young Min; Jeong, Jee Hyang; Park, Kyung Won; Song, Min Kyung; Lee, Ho Won (2021). "Potential novel genes for late-onset alzheimer's disease in east-asian descent identified by APOE-Stratified genome-wide association study". Journal of Alzheimer's Disease. 82 (4): 1451–1460. doi:10.3233/JAD-210145. ISSN 1387-2877.
- ^ "GCH1 GTP cyclohydrolase 1 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2023-12-03.
- ^ a b Marucci, Gabriella; Buccioni, Michela; Ben, Diego Dal; Lambertucci, Catia; Volpini, Rosaria; Amenta, Francesco (2021-06-01). "Efficacy of acetylcholinesterase inhibitors in Alzheimer's disease". Neuropharmacology. 190: 108352. doi:10.1016/j.neuropharm.2020.108352. ISSN 1873-7064. PMID 33035532.
- ^ Hao, Xiaoyan; Wang, Aijun; Li, Chong; Shao, Lufei; Li, Yi; Yang, Ping (2021-02). "Genetic association of BIN1 and GAB2 in Alzheimer's disease: A meta‐analysis and systematic review". Geriatrics & Gerontology International. 21 (2): 185–191. doi:10.1111/ggi.14109. ISSN 1444-1586. PMC 7898709. PMID 33331110.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ a b c Hampel, H., Hardy, J., Blennow, K. et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol Psychiatry 26, 5481–5503 (2021). https://doi.org/10.1038/s41380-021-01249-0
- ^ Eysert, Fanny; Coulon, Audrey; Boscher, Emmanuelle; Vreulx, Anaїs-Camille; Flaig, Amandine; Mendes, Tiago; Hughes, Sandrine; Grenier-Boley, Benjamin; Hanoulle, Xavier; Demiautte, Florie; Bauer, Charlotte; Marttinen, Mikael; Takalo, Mari; Amouyel, Philippe; Desai, Shruti (2021-10). "Alzheimer's genetic risk factor FERMT2 (Kindlin-2) controls axonal growth and synaptic plasticity in an APP-dependent manner". Molecular Psychiatry. 26 (10): 5592–5607. doi:10.1038/s41380-020-00926-w. ISSN 1476-5578.
{{cite journal}}: Check date values in:|date=(help) - ^ Ly, Philip T. T.; Wu, Yili; Zou, Haiyan; Wang, Ruitao; Zhou, Weihui; Kinoshita, Ayae; Zhang, Mingming; Yang, Yi; Cai, Fang; Woodgett, James; Song, Weihong (2013-01-02). "Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes". The Journal of Clinical Investigation. 123 (1): 224–235. doi:10.1172/JCI64516. ISSN 0021-9738. PMID 23202730.
- ^ a b Thomas, Rhian S.; Henson, Alex; Gerrish, Amy; Jones, Lesley; Williams, Julie; Kidd, Emma J. (2016-07-18). "Decreasing the expression of PICALM reduces endocytosis and the activity of β-secretase: implications for Alzheimer's disease". BMC Neuroscience. 17 (1): 50. doi:10.1186/s12868-016-0288-1. ISSN 1471-2202. PMC 4949774. PMID 27430330.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Eisenmenger, Laura B.; Peret, Anthony; Famakin, Bolanle M.; Spahic, Alma; Roberts, Grant S.; Bockholt, Jeremy H.; Johnson, Kevin M.; Paulsen, Jane S. (2023-04). "Vascular contributions to Alzheimer's disease". Translational Research: The Journal of Laboratory and Clinical Medicine. 254: 41–53. doi:10.1016/j.trsl.2022.12.003. ISSN 1878-1810. PMID 36529160.
{{cite journal}}: Check date values in:|date=(help) - ^ "EXOC3L2 exocyst complex component 3 like 2 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2023-12-03.
- ^ a b c d Wu, Kai-Min; Zhang, Ya-Ru; Huang, Yu-Yuan; Dong, Qiang; Tan, Lan; Yu, Jin-Tai (2021-09-01). "The role of the immune system in Alzheimer's disease". Ageing Research Reviews. 70: 101409. doi:10.1016/j.arr.2021.101409. ISSN 1568-1637.
- ^ "KCNJ15 potassium inwardly rectifying channel subfamily J member 15 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2023-12-03.
- ^ "HLA-DRB1 major histocompatibility complex, class II, DR beta 1 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2023-12-03.
- ^ a b c Song, Limin; Pei, Lei; Hu, Lisha; Pan, Shangwen; Xiong, Wei; Liu, Min; Wu, Yan; Shang, You; Yao, Shanglong (2018-07-02). "Death-associated protein kinase 1 mediates interleukin-1β production through regulating inlfammasome activation in Bv2 microglial cells and mice". Scientific Reports. 8 (1): 9930. doi:10.1038/s41598-018-27842-y. ISSN 2045-2322.
- ^ Griciuc, Ana; Serrano-Pozo, Alberto; Parrado, Antonio R.; Lesinski, Andrea N.; Asselin, Caroline N.; Mullin, Kristina; Hooli, Basavaraj; Choi, Se Hoon; Hyman, Bradley T.; Tanzi, Rudolph E. (2013-05-22). "Alzheimer's Disease Risk Gene CD33 Inhibits Microglial Uptake of Amyloid Beta". Neuron. 78 (4): 631–643. doi:10.1016/j.neuron.2013.04.014. ISSN 0896-6273.
- ^ a b c Proitsi, Petroula; Lee, Sang Hyuck; Lunnon, Katie; Keohane, Aoife; Powell, John; Troakes, Claire; Al-Sarraj, Safa; Furney, Simon; Soininen, Hilkka; Kłoszewska, Iwona; Mecocci, Patrizia; Tsolaki, Magda; Vellas, Bruno; Lovestone, Simon; Hodges, Angela (2014-02-01). "Alzheimer's disease susceptibility variants in the MS4A6A gene are associated with altered levels of MS4A6A expression in blood". Neurobiology of Aging. 35 (2): 279–290. doi:10.1016/j.neurobiolaging.2013.08.002. ISSN 0197-4580.
- ^ a b c Lee, Sang E.; Lee, Hee Yun; Diwan, Sadhna (2010-01). "What do Korean American immigrants know about Alzheimer's disease (AD)? The impact of acculturation and exposure to the disease on AD knowledge". International Journal of Geriatric Psychiatry. 25 (1): 66–73. doi:10.1002/gps.2299. ISSN 1099-1166. PMID 19551701.
{{cite journal}}: Check date values in:|date=(help) - ^ Lee, Hochang Benjamin; Han, Hae-Ra; Huh, Bo-Yun; Kim, Kim B.; Kim, Miyong T. (2014). "Mental health service utilization among Korean elders in Korean churches: preliminary findings from the Memory and Aging Study of Koreans in Maryland (MASK-MD)". Aging & Mental Health. 18 (1): 102–109. doi:10.1080/13607863.2013.814099. ISSN 1364-6915. PMC 4519089. PMID 23889338.
- ^ a b c Chow, T. W.; Liu, C. K.; Fuh, J. L.; Leung, V. P. Y.; Tai, C. T.; Chen, Li-Wen; Wang, S. J.; Chiu, H. F. K.; Lam, L. C. W.; Chen, Q. L.; Cummings, J. L. (2002-01). "Neuropsychiatric symptoms of Alzheimer's disease differ in Chinese and American patients". International Journal of Geriatric Psychiatry. 17 (1): 22–28. doi:10.1002/gps.509. ISSN 0885-6230. PMID 11802226.
{{cite journal}}: Check date values in:|date=(help) - ^ Braun, K. L.; Takamura, J. C.; Mougeot, T. (1996-09). "Perceptions of dementia, caregiving, and help-seeking among recent Vietnamese immigrants". Journal of Cross-Cultural Gerontology. 11 (3): 213–228. doi:10.1007/BF00122702. ISSN 0169-3816. PMID 24390036.
{{cite journal}}: Check date values in:|date=(help) - ^ Jones, Randi S.; Chow, Tiffany W.; Gatz, Margaret (2006-01-01). "Asian Americans and Alzheimer's disease: Assimilation, culture, and beliefs". Journal of Aging Studies. 20 (1): 11–25. doi:10.1016/j.jaging.2005.01.001. ISSN 0890-4065.
- ^ Hinton, Ladson; Guo, Zibin; Hillygus, Jennifer; Levkoff, Sue (2000). Journal of Cross-Cultural Gerontology. 15 (2): 119–137. doi:10.1023/a:1006798316654. ISSN 0169-3816 http://dx.doi.org/10.1023/a:1006798316654.
{{cite journal}}: Missing or empty|title=(help) - ^ a b Hinton, Ladson; Franz, Carol E.; Yeo, Gwen; Levkoff, Sue E. (2005-08). "Conceptions of dementia in a multiethnic sample of family caregivers". Journal of the American Geriatrics Society. 53 (8): 1405–1410. doi:10.1111/j.1532-5415.2005.53409.x. ISSN 0002-8614. PMID 16078970.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Guo Z, Levy BR, Hinton WL, Weitzman PF, Levkoff SE. 2000. The poser of labels: recruiting dementia-affected Chinese American elders and their caregivers. J Ment Health Aging 6: 103–112.
- ^ a b Yuri Jang, null; Kim, Giyeon; Chiriboga, David (2010-06). "Knowledge of Alzheimer's disease, feelings of shame, and awareness of services among Korean American elders". Journal of Aging and Health. 22 (4): 419–433. doi:10.1177/0898264309360672. ISSN 1552-6887. PMC 2882867. PMID 20194682.
{{cite journal}}: Check date values in:|date=(help) - ^ Hirakawa, Yoshihisa; Chiang, Chifa; Aoyama, Atsuko (2017-05). "A qualitative study on barriers to achieving high-quality, community-based integrated dementia care". Journal of rural medicine: JRM. 12 (1): 28–32. doi:10.2185/jrm.2927. ISSN 1880-487X. PMC 5458349. PMID 28593014.
{{cite journal}}: Check date values in:|date=(help) - ^ Tham, Tat Yean; Tran, Thuy Linh; Prueksaritanond, Somjit; Isidro, Josefina S.; Setia, Sajita; Welluppillai, Vicknesh (2018). "Integrated health care systems in Asia: an urgent necessity". Clinical Interventions in Aging. 13: 2527–2538. doi:10.2147/CIA.S185048. ISSN 1178-1998. PMC 6298881. PMID 30587945.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Mattke, Soeren; Loh, Wei Kok; Yuen, Kah‐Hung; Yoong, Joanne (2023-06-06). "Preparedness of China's health care system to provide access to a disease‐modifying Alzheimer's treatment". Alzheimer's & Dementia. doi:10.1002/alz.13348. ISSN 1552-5260.
- ^ Mattke, Soeren; Loh, Wei Kok; Yuen, Kah‐Hung; Yoong, Joanne (2023-06-06). "Preparedness of China's health care system to provide access to a disease‐modifying Alzheimer's treatment". Alzheimer's & Dementia. doi:10.1002/alz.13348. ISSN 1552-5260.
- ^ a b c Sun, Fei; Gao, Xiang; Shen, Hui; Burnette, Denise (2014-06). "Levels and correlates of knowledge about Alzheimer's disease among older Chinese Americans". Journal of Cross-Cultural Gerontology. 29 (2): 173–183. doi:10.1007/s10823-014-9229-6. ISSN 1573-0719. PMID 24728621.
{{cite journal}}: Check date values in:|date=(help) - ^ Roberts, J. S.; Connell, C. M. (2000). "Illness representations among first-degree relatives of people with Alzheimer disease". Alzheimer Disease and Associated Disorders. 14 (3): 129–136, Discussion 127–128. doi:10.1097/00002093-200007000-00003. ISSN 0893-0341. PMID 10994653.
- ^ Carpenter, Brian D.; Zoller, Sarah M.; Balsis, Steve; Otilingam, Poorni G.; Gatz, Margaret (2011-03). "Demographic and contextual factors related to knowledge about Alzheimer's disease". American Journal of Alzheimer's Disease and Other Dementias. 26 (2): 121–126. doi:10.1177/1533317510394157. ISSN 1938-2731. PMC 4441262. PMID 21233137.
{{cite journal}}: Check date values in:|date=(help) - ^ Ayalon, Liat; Areán, Patricia A. (2004-01). "Knowledge of Alzheimer's disease in four ethnic groups of older adults". International Journal of Geriatric Psychiatry. 19 (1): 51–57. doi:10.1002/gps.1037. ISSN 0885-6230. PMID 14716699.
{{cite journal}}: Check date values in:|date=(help) - ^ Wang DS (2012) Caregiving for dementia in Asian communities: Implications for practice. Journal of Ethnic & Cultural Diversity in Social Work: Innovation in Theory, Research & Practice 21, 249–273
- ^ Jones, Randi S.; Chow, Tiffany W.; Gatz, Margaret (2006-01-01). "Asian Americans and Alzheimer's disease: Assimilation, culture, and beliefs". Journal of Aging Studies. 20 (1): 11–25. doi:10.1016/j.jaging.2005.01.001. ISSN 0890-4065.
- ^ Jack, Clifford R.; Bennett, David A.; Blennow, Kaj; Carrillo, Maria C.; Dunn, Billy; Haeberlein, Samantha Budd; Holtzman, David M.; Jagust, William; Jessen, Frank; Karlawish, Jason; Liu, Enchi; Molinuevo, Jose Luis; Montine, Thomas; Phelps, Creighton; Rankin, Katherine P. (2018-04). "NIA‐AA Research Framework: Toward a biological definition of Alzheimer's disease". Alzheimer's & Dementia. 14 (4): 535–562. doi:10.1016/j.jalz.2018.02.018. ISSN 1552-5260. PMC 5958625. PMID 29653606.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Jack, Clifford R.; Bennett, David A.; Blennow, Kaj; Carrillo, Maria C.; Dunn, Billy; Haeberlein, Samantha Budd; Holtzman, David M.; Jagust, William; Jessen, Frank; Karlawish, Jason; Liu, Enchi; Molinuevo, Jose Luis; Montine, Thomas; Phelps, Creighton; Rankin, Katherine P. (2018-04). "NIA‐AA Research Framework: Toward a biological definition of Alzheimer's disease". Alzheimer's & Dementia. 14 (4): 535–562. doi:10.1016/j.jalz.2018.02.018. ISSN 1552-5260. PMC 5958625. PMID 29653606.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Cai, Huimin; Pang, Yana; Fu, Xiaofeng; Ren, Ziye; Jia, Longfei (2023-10-24). "Plasma biomarkers predict Alzheimer's disease before clinical onset in Chinese cohorts". Nature Communications. 14 (1): 6747. doi:10.1038/s41467-023-42596-6. ISSN 2041-1723.
- ^ a b c d e Cai, Huimin; Pang, Yana; Fu, Xiaofeng; Ren, Ziye; Jia, Longfei (2023-10-24). "Plasma biomarkers predict Alzheimer's disease before clinical onset in Chinese cohorts". Nature Communications. 14 (1): 6747. doi:10.1038/s41467-023-42596-6. ISSN 2041-1723.
- ^ Xue Wu, Zhenxu Xiao, Jingwei Yi, Saineng Ding, Hongchen Gu, Wanqing Wu, Jianfeng Luo, Xiaoniu Liang, Li Zheng, Hong Xu, Qianhua Zhao, Ding Ding, Development of a Plasma Biomarker Diagnostic Model Incorporating Ultrasensitive Digital Immunoassay as a Screening Strategy for Alzheimer Disease in a Chinese Population, Clinical Chemistry, Volume 67, Issue 12, December 2021, Pages 1628–1639, https://doi.org/10.1093/clinchem/hvab192
- ^ Cullen, Nicholas C.; Leuzy, Antoine; Janelidze, Shorena; Palmqvist, Sebastian; Svenningsson, Anna L.; Stomrud, Erik; Dage, Jeffrey L.; Mattsson-Carlgren, Niklas; Hansson, Oskar (2021-06-11). "Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations". Nature Communications. 12 (1): 3555. doi:10.1038/s41467-021-23746-0. ISSN 2041-1723. PMC 8196018. PMID 34117234.
- ^ Cai, Huimin; Pang, Yana; Fu, Xiaofeng; Ren, Ziye; Jia, Longfei (2023-10-24). "Plasma biomarkers predict Alzheimer's disease before clinical onset in Chinese cohorts". Nature Communications. 14 (1): 6747. doi:10.1038/s41467-023-42596-6. ISSN 2041-1723.
- ^ a b Wilkins, Consuelo H.; Windon, Charles C.; Dilworth-Anderson, Peggye; Romanoff, Justin; Gatsonis, Constantine; Hanna, Lucy; Apgar, Charles; Gareen, Ilana F.; Hill, Carl V.; Hillner, Bruce E.; March, Andrew; Siegel, Barry A.; Whitmer, Rachel A.; Carrillo, Maria C.; Rabinovici, Gil D. (2022-11-01). "Racial and Ethnic Differences in Amyloid PET Positivity in Individuals With Mild Cognitive Impairment or Dementia: A Secondary Analysis of the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) Cohort Study". JAMA Neurology. 79 (11): 1139–1147. doi:10.1001/jamaneurol.2022.3157. ISSN 2168-6149.
- ^ Mattke, Soeren; Loh, Wei Kok; Yuen, Kah‐Hung; Yoong, Joanne (2023-06-06). "Preparedness of China's health care system to provide access to a disease‐modifying Alzheimer's treatment". Alzheimer's & Dementia. doi:10.1002/alz.13348. ISSN 1552-5260.
- ^ Imaging, Life Molecular. "Life Molecular Imaging and Sinotau Pharmaceutical Group Announce the Regulatory Approval of their Amyloid PET Imaging Radiopharmaceutical Neuraceq® in China". www.prnewswire.com. Retrieved 2023-12-06.
