User:Benjah-bmm27/Structure types
Appearance
AB structures
[edit]Halite (rock salt) structure
[edit]LiF, LiCl, LiBr, LiI, NaF, NaCl, NaBr, NaI, KF, KCl, KBr, KI, RbF, RbCl, RbBr, RbI, CsF, MgO, PbS, AgF, AgCl, AgBr
Caesium chloride structure
[edit]CsCl, CsBr, CsI, high-temp RbCl, AlCo, AgZn, BeCu, MgCe, RuAl, SrTl.
Sphalerite (zinc blende) structure
[edit]β-ZnS, α-AgI, β-BN, CuBr, β-CdS, BP, BAs
If A = B, the sphalerite structure becomes the diamond structure.
Wurtzite structure
[edit]α-ZnS, β-AgI, ZnO, α-CdS, CdSe, α-SiC, GaN, AlN, w-BN, BeO
AB2 structures
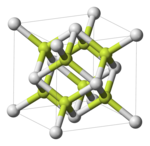
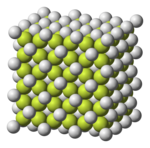
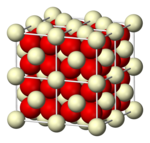
[edit]Fluorite structure
[edit]CaF2, BaF2, CdF2, HgF2, SrF2, SrCl2, CeO2, ThO2, ZrO2
 |
 |
 |
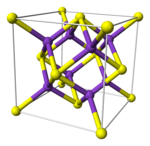
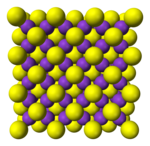
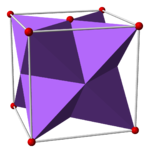
Antifluorite structure
[edit]Alkali metal chalcogenides, M2X, M = Li, Na, K, Rb; X = O, S, Se, Te
Li2O, Li2S, Li2Se, Li2Te, Na2O, Na2S, Na2Se, Na2Te, K2O, K2S, K2Se, K2Te, Rb2O, Rb2S, Rb2Se
 |
 |
 |
 | |
Rutile structure
[edit]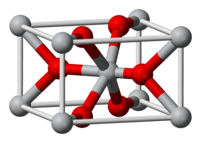
Antirutile structure
[edit]- ε-Ti2N
Cadmium chloride structure
[edit]Cadmium iodide structure
[edit]Iodides
[edit]MgI2, TiI2, VI2, MnI2, FeI2, CoI2, CaI2, PdI2, PbI2.
Chlorides and bromides
[edit]MgBr2, TiBr2, VBr2, MnBr2, FeBr2, CoBr2.
Hydroxides of M2+
[edit]Chalcogenides of M4+
[edit]TiS2, ZrS2, SnS2, α-TaS2, PtS2;
SiTe2, TiTe2, CoTe2, NiTe2, PdTe2, PtTe2.
