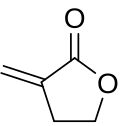
Tulipalin A
Appearance
| |
| Names | |
|---|---|
| IUPAC name
α-methylene-γ-butyrolactone
| |
| Preferred IUPAC name
3-Methylideneoxolan-2-one | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 107939 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.120 |
| EC Number |
|
| 746139 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H6O2 | |
| Molar mass | 98.101 g·mol−1 |
| Density | 1.085 g/ml |
| Melting point | 25 °C (77 °F; 298 K) |
| Boiling point | 168 °C (334 °F; 441 K) |
| Soluble in organic solvents like acetone and slightly soluble in water | |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H226, H317 | |
| P210, P233, P240, P241, P242, P243, P261, P272, P280, P302+P352, P303+P361+P353, P321, P333+P313, P363, P370+P378, P403+P235, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tulipalin A, also known as α-methylene-γ-butyrolactone,[1] is a naturally occurring compound found in certain flowers such as tulips and alstroemerias.[2] Tulipalin A has the molecular formula C5H6O2 and the CAS registry number 547-65-9.[1] It is an allergen and has been known to cause occupational contact dermatitis, i.e. 'tulip fingers,' in some who are commonly exposed to it such as florists.[3] It has been shown to be synthesized from Tuliposide-A in response to damages to the plant. When the plant is damaged, Tuliposide-A is broken down by Tuliposide-converting enzymes (TCE) to produce Tulipalin-A. More recent experiments with this compound have uncovered potential applications for it in the field of polymerization.[4][5]
References
[edit]- ^ a b CID 68352 from PubChem
- ^ Christensen, Lars P. (1999). "Direct release of the allergen tulipalin a from Alstroemeriacut flowers: A possible source of airborne contact dermatitis?". Contact Dermatitis. 41 (6): 320–324. doi:10.1111/j.1600-0536.1999.tb06180.x. PMID 10617212. S2CID 23478644.
- ^ McCluskey, J.; Bourgeois, M.; Harbison, R. (2014). "Tulipalin a induced phytotoxicity". International Journal of Critical Illness and Injury Science. 4 (2): 181–183. doi:10.4103/2229-5151.134187. PMC 4093970. PMID 25024947.
- ^ Zhou, Jiawen; Schmidt, Annette M.; Ritter, Helmut (2010). "Bicomponent Transparent Polyester Networks with Shape Memory Effect". Macromolecules. 43 (2): 939–942. Bibcode:2010MaMol..43..939Z. doi:10.1021/ma901402a.
- ^ Shin, Jihoon; Lee, Youngmin; Tolman, William B.; Hillmyer, Marc A. (2012). "Thermoplastic Elastomers Derived from Menthide and Tulipalin A". Biomacromolecules. 13 (11): 3833–3840. doi:10.1021/bm3012852. PMID 23062206.
