Thalidomide scandal
| Phocomelia | |
|---|---|
 | |
| Cases of severe thalidomide-induced phocomelia |
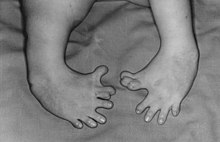
In the late 1950s and early 1960s, the use of thalidomide in 46 countries was prescribed to women who were pregnant or who subsequently became pregnant, and consequently resulted in the "biggest anthropogenic medical disaster ever," with more than 10,000 children born with a range of severe deformities, such as phocomelia, as well as thousands of miscarriages.[1][2]
Thalidomide was introduced in 1953 as a tranquilizer, and was later marketed by the German pharmaceutical company Chemie Grünenthal under the trade name Contergan as a medication for anxiety, trouble sleeping, tension, and morning sickness.[3][4] It was introduced as a sedative and medication for morning sickness without having been tested on pregnant women.[5] While initially deemed to be safe in pregnancy, concerns regarding birth defects were noted in 1961, and the medication was removed from the market in Europe that year.[3][6]
Development of thalidomide
[edit]
Thalidomide was first developed as a tranquilizer by Swiss pharmaceutical company Ciba in 1953. In 1954, Ciba abandoned the product, and it was acquired by German pharmaceutical company Chemie Grünenthal.[4] The company had been established by Hermann Wirtz Sr, a Nazi Party member, after World War II as a subsidiary of the family's Mäurer & Wirtz company. The company's initial aim was to develop antibiotics for which there was an urgent market need. Wirtz appointed chemist Heinrich Mückter, who had escaped prosecution for war crimes for his experiments on prisoners of Nazi concentration camps, to head the development programme because of his experience researching and producing an anti-typhus vaccine for Nazi Germany.[7] He hired Martin Staemmler, a medical doctor and leading proponent of the Nazi eugenics programme, as head of pathology, as well as Heinz Baumkötter, the chief medical officer at the Sachsenhausen concentration camp, and Otto Ambros, a chemist and Nazi war criminal. Ambros was the chairman of Grünenthal's advisory committee during the development of thalidomide and was a board member when Contergan was being sold.[8]
Birth defect crisis
[edit]The total number of embryos affected by the use of thalidomide during pregnancy is estimated at more than 10,000, and potentially up to 20,000; of these, approximately 40 percent died at or shortly after the time of birth.[3][9][10] Those who survived had limb, eye, urinary tract, and heart defects.[6] Its initial entry into the U.S. market was prevented by Frances Oldham Kelsey at the U.S. Food and Drug Administration (FDA).[4] The birth defects of thalidomide led to the development of greater drug regulation and monitoring in many countries.[4][6]
The severity and location of the deformities depended on how many days into the pregnancy the mother was before beginning treatment; thalidomide taken on the 20th day of pregnancy caused central brain damage, day 21 would damage the eyes, day 22 the ears and face, day 24 the arms, and leg damage would occur if taken up to day 28. Thalidomide did not damage the fetus if taken after 42 days' gestation.[11][12]
United Kingdom
[edit]
In the UK, the drug was licensed in 1958 and withdrawn in 1961. Of the approximately 2,000 babies born with defects, around half died within a few months and 466 survived to at least 2010.[13] In 1968, after a long campaign by The Sunday Times, a compensation settlement for the UK victims was reached with Distillers Company (now part of Diageo), which had distributed the drug in the UK.[14][15] Distillers Biochemicals paid out approximately £28m in compensation following a legal battle.[16]
The British Thalidomide Children's Trust was set up in 1973 as part of a £20 million legal settlement between Distillers Company and 429 children with thalidomide-related disabilities. In 1997, Diageo (formed by a merger between Grand Metropolitan and Guinness, who had taken over Distillers in 1990) made a long-term financial commitment to support the Thalidomide Trust and its beneficiaries.[17] The UK government gave survivors a grant of £20 million, to be distributed through the Thalidomide Trust, in December 2009.[16]
Spain
[edit]In Spain, thalidomide was widely available throughout the 1970s, and perhaps even into the 1980s. There were two reasons for this. First, state controls and safeguarding were poor; it was not until 2008 that the government even admitted the country had ever imported thalidomide. Second, Grünenthal failed to insist that its sister company in Madrid warn Spanish doctors, and permitted its sister company not to warn doctors of the defects. The Spanish advocacy group for victims of thalidomide estimates that in 2015, there were 250–300 living victims of thalidomide in Spain.[18]
Australia and New Zealand
[edit]Although the Australian obstetrician William McBride took credit for raising concern about thalidomide, it was a midwife called Sister Pat Sparrow who first suspected the drug was causing birth defects in the babies of patients under McBride's care at Crown Street Women's Hospital in Sydney.[19] German paediatrician Widukind Lenz, who also suspected the link, is credited with conducting the scientific research that proved thalidomide was causing birth defects in 1961.[20][21] McBride was later awarded a number of honors, including a medal and prize money by L'Institut de la Vie in Paris,[22] but he was eventually struck off the Australian medical register in 1993 for scientific fraud related to work on Debendox.[19][23] Further animal tests were conducted by George Somers, Chief Pharmacologist of Distillers Company in Britain, which showed fetal abnormalities in rabbits.[24] Similar results were also published showing these effects in rats[25][26] and other species.[27]
Lynette Rowe, who was born without limbs, led an Australian class action lawsuit against the drug's manufacturer, Grünenthal, which fought to have the case heard in Germany. The Supreme Court of Victoria dismissed Grünenthal's application in 2012, and the case was heard in Australia.[28] On 17 July 2012, Rowe was awarded an out-of-court settlement, believed to be in the millions of dollars and providing precedence for class action victims to receive further compensation.[29] In February 2014, the Supreme Court of Victoria endorsed the settlement of $89 million AUD to 107 victims of the drug in Australia and New Zealand.[30][31]
Germany
[edit]In East Germany, thalidomide was rejected by the Central Committee of Experts for the Drug Traffic in the GDR, and was never approved for use. There are no known thalidomide babies born in East Germany.[32] Meanwhile, in West Germany, it took some time before the increase in dysmelia at the end of the 1950s was connected with thalidomide. In 1958, Karl Beck, a former pediatric doctor in Bayreuth, wrote an article in a local newspaper claiming a relationship between nuclear weapons testing and cases of dysmelia in children.[33] Based on this, FDP whip Erich Mende requested an official statement from the federal government.[33] For statistical reasons, the main data series used to research dysmelia cases started by chance at the same time as the approval date for thalidomide.[33] After the Nazi regime with its Law for the Prevention of Hereditarily Diseased Offspring used mandatory statistical monitoring to commit various crimes, western Germany had been very reluctant to monitor congenital disorders in a similarly strict way.[34] The parliamentary report rejected any relation with radioactivity and the abnormal increase of dysmelia.[33] Also the DFG research project installed after the Mende request was not helpful. The project was led by pathologist Franz Büchner, who ran the project to propagate his teratological theory. Büchner saw lack of healthy nutrition and behavior of the mothers as being more important than genetic reasons.[34] Furthermore, it took a while to appoint a Surgeon General in Germany; the Federal Ministry of Health was not founded until 1962, some months after thalidomide was banned from the market.[33] In West Germany approximately 2,500 babies were born with birth defects from thalidomide.[21]
Canada
[edit]Despite its severe side effects, thalidomide was sold in pharmacies in Canada until 1962.[35][36] The effects of thalidomide increased fears regarding the safety of pharmaceutical drugs. The Society of Toxicology of Canada was formed after the effects of thalidomide were made public, focusing on toxicology as a discipline separate from pharmacology.[37] The need for the testing and approval of the toxins in certain pharmaceutical drugs became more important after the disaster. The Society of Toxicology of Canada is responsible for the Conservation Environment Protection Act, focusing on researching the impact to human health of chemical substances.[37] Thalidomide brought on changes in the way drugs are tested, what type of drugs are used during pregnancy, and increased the awareness of potential side effects of drugs.
According to Canadian news magazine programme W5, most, but not all, victims of thalidomide receive annual benefits as compensation from the Government of Canada. Excluded are those who cannot provide the documentation the government requires.[38]
A group of 120 Canadian survivors formed the Thalidomide Victims Association of Canada, the goal of which is to prevent the approval of drugs that could be harmful to pregnant individuals and babies.[39][40] The members from the thalidomide victims association were involved in the STEPS programme, which aimed to prevent teratogenicity.[41]
United States
[edit]
In the U.S., the FDA refused approval to market thalidomide, saying further studies were needed. This reduced the impact of thalidomide in U.S. patients. The refusal was largely due to pharmacologist Frances Oldham Kelsey who withstood pressure from the Richardson-Merrell Pharmaceuticals Co. Although thalidomide was not approved for sale in the United States at the time, over 2.5 million tablets had been distributed to over 1,000 physicians during a clinical testing programme. It is estimated that nearly 20,000 patients, several hundred of whom were pregnant, were given the drug to help alleviate morning sickness or as a sedative, and at least 17 children were consequently born in the United States with thalidomide-associated deformities.[42][43] While pregnant, children's television host Sherri Finkbine took thalidomide that her husband had purchased over-the-counter in Europe.[44] When she learned that thalidomide was causing fetal deformities she wanted to abort her pregnancy, but the laws of Arizona allowed abortion only if the mother's life was in danger. Finkbine traveled to Sweden to have the abortion. Thalidomide was found to have deformed the fetus.[42]
For denying the application despite the pressure from Richardson-Merrell Pharmaceuticals Co., Kelsey eventually received the President's Award for Distinguished Federal Civilian Service at a 1962 ceremony with President John F. Kennedy.[2] In September 2010, the FDA honored Kelsey with the first Kelsey award, given annually to an FDA staff member. This came 50 years after Kelsey, then a new medical officer at the agency, first reviewed the application from the William S. Merrell Pharmaceuticals Company of Cincinnati.[45]
Cardiologist Helen B. Taussig learned of the damaging effects of the drug thalidomide on newborns and in 1967, testified before Congress on this matter after a trip to Germany where she worked with infants with phocomelia (severe limb deformities). As a result of her efforts, thalidomide was banned in the United States and Europe.[46]
Austria
[edit]Ingeborg Eichler, a member of the Austrian pharmaceutical admission conference, enforced restrictions on the sale of thalidomide (tradename Softenon) under the rules of prescription medication and as a result relatively few affected children were born in Austria and Switzerland.[47]
Aftermath of scandal
[edit]
The numerous reports of malformations in babies brought about the awareness of the side effects of the drug on pregnant women. The birth defects caused by the drug thalidomide can range from moderate malformation to more severe forms. Possible birth defects include phocomelia, dysmelia, amelia, bone hypoplasticity, and other congenital defects affecting the ear, heart, or internal organs.[41] Franks et al. looked at how the drug affected newborn babies, the severity of their deformities, and reviewed the drug in its early years. Webb in 1963 also reviewed the history of the drug and the different forms of birth defects it had caused. "The most common form of birth defects from thalidomide is shortened limbs, with the arms being more frequently affected. This syndrome is the presence of deformities of the long bones of the limbs resulting in shortening and other abnormalities."[35]
Grünenthal criminal trial
[edit]In 1968, a large criminal trial began in West Germany, charging several Grünenthal officials with negligent homicide and injury. After Grünenthal settled with the victims in April 1970, the trial ended in December 1970 with no finding of guilt. As part of the settlement, Grünenthal paid 100 million DM into a special foundation; the West German government added 320 million DM. The foundation paid victims a one-time sum of 2,500–25,000 DM (depending on severity of disability) and a monthly stipend of 100–450 DM. The monthly stipends have since been raised substantially and are now paid entirely by the government (as the foundation had run out of money). Grünenthal paid another €50 million into the foundation in 2008.
On 31 August 2012, Grünenthal chief executive Harald F. Stock— who served as the chief executive officer of Grünenthal GmbH from January 2009 to May 28, 2013— apologized for the first time for producing the drug and remaining silent about the birth defects.[48] At a ceremony, Stock unveiled a statue of a disabled child to symbolize those harmed by thalidomide and apologized for not trying to reach out to victims for over 50 years. At the time of the apology, there were between 5,000 and 6,000 people still living with Thalidomide-related birth defects. Victim advocates called the apology "insulting" and "too little, too late", and criticized the company for not compensating victims and for their claim that no one could have known the harm the drug caused, arguing that there were plenty of red flags at the time.[49]
Australian National Memorial
[edit]On 13 November 2023, the Australian Government announced its intention to make a formal apology to people affected by thalidomide with the unveiling of a national memorial site. Prime Minister Anthony Albanese described the thalidomide tragedy as a “dark chapter” in Australian history, and Health Minister Mark Butler said, “While we cannot change the past or end the physical suffering, I hope these important next steps of recognition and apology will help heal some of the emotional wounds.”[50][51]
Notable cases
[edit]
- Mercédes Benegbi, born with phocomelia of both arms, drove the successful campaign for compensation from her government for Canadians who were affected by thalidomide.[52]
- Mat Fraser, born with phocomelia of both arms, is an English rock musician, actor, writer and performance artist. He produced a 2002 television documentary, Born Freak, which looked at this historical tradition and its relevance to modern disabled performers. This work has become the subject of academic analysis in the field of disability studies.[53]
- Niko von Glasow, a thalidomide survivor, produced a documentary called NoBody's Perfect, based on the lives of 12 people affected by the drug, which was released in 2008.[54][55]
- Josée Lake is a Canadian Paralympic gold medallist swimmer, thalidomide survivor, and president of the Thalidomide Victims Association of Canada
- Lorraine Mercer MBE of the United Kingdom, born with phocomelia of both arms and legs, is the only thalidomide survivor to carry the Olympic Torch.[56]
- Thomas Quasthoff, an internationally acclaimed bass-baritone, who describes himself: "1.34 meters tall, short arms, seven fingers — four right, three left — large, relatively well-formed head, brown eyes, distinctive lips; profession: singer".[57]
- Alvin Law, Canadian motivational speaker and former radio broadcaster.
Change in drug regulations
[edit]The disaster prompted many countries to introduce tougher rules for the testing and licensing of drugs, such as the Kefauver Harris Amendment[58] (US), Directive 65/65/EEC1 (E.U.),[59] and the Medicines Act 1968 (UK).[60][61] In the United States, the new regulations strengthened the FDA, among other ways, by requiring applicants to prove efficacy and to disclose all side effects encountered in testing.[2] The FDA subsequently initiated the Drug Efficacy Study Implementation to reclassify drugs already on the market.
References
[edit]- ^ Vargesson, Neil (June 2015). "Thalidomide-induced teratogenesis: history and mechanisms". Birth Defects Research Part C: Embryo Today: Reviews. 105 (2): 140–156. doi:10.1002/bdrc.21096. ISSN 1542-9768. PMC 4737249. PMID 26043938.
- ^ a b c Bren L (28 February 2001). "Frances Oldham Kelsey: FDA Medical Reviewer Leaves Her Mark on History". FDA Consumer. U.S. Food and Drug Administration. Retrieved 23 December 2009.
- ^ a b c Miller MT (1991). "Thalidomide Embryopathy: A Model for the Study of Congenital Incomitant Horizontal Strabismus". Transactions of the American Ophthalmological Society. 81: 623–674. PMC 1298636. PMID 1808819.
- ^ a b c d Loue S, Sajatovic M (2004). Encyclopedia of Women's Health. Springer Science & Business Media. pp. 643–644. ISBN 9780306480737.
- ^ Sneader W (2005). Drug discovery: a history (Rev. and updated ed.). Chichester: Wiley. p. 367. ISBN 978-0-471-89979-2.
- ^ a b c Cuthbert A (2003). The Oxford Companion to the Body. Oxford University Press. p. 682. doi:10.1093/acref/9780198524038.001.0001. ISBN 9780198524038.
- ^ Thomas, Katie (2020-03-23). "The Unseen Survivors of Thalidomide Want to Be Heard". The New York Times. ISSN 0362-4331. Retrieved 2020-03-23.
- ^ Williams, Roger (September 10, 2012). "The Nazis and Thalidomide: The Worst Drug Scandal of All Time". Newsweek. Retrieved 25 August 2021.
- ^ "Thalidomide Monograph for Professionals". Drugs.com. Retrieved 14 November 2019.
- ^ Zimmer C (15 March 2010). "Answers Begin to Emerge on How Thalidomide Caused Defects". New York Times. Retrieved 2010-03-21.
As they report in the current issue of Science, a protein known as cereblon latched on tightly to the thalidomide
- ^ "Thalidomide: The Fifty Year Fight (no longer available)". BBC. 15 May 2014. Retrieved 13 September 2015.[dead YouTube link]
- ^ Miller M. T., Strömland K. (November 1999). "Teratogen update: Thalidomide: a review, with a focus on ocular findings and new potential uses" (PDF).
- ^ "Apology for thalidomide survivors". BBC News. 14 January 2010. Retrieved 14 January 2010.
- ^ Ryan C (1 April 2004). "They just didn't know what it would do". BBC News:Health. BBC News. Archived from the original on 2004-07-07. Retrieved 1 May 2009.
- ^ Flintoff JP (23 March 2008). "Thalidomide: the battle for compensation goes on". The Sunday Times. London: Times Newspapers Ltd. Archived from the original on 2008-05-13. Retrieved 2009-05-01.
- ^ a b "Thalidomide survivors to get £20m". BBC News. 23 December 2009. Archived from the original on 14 January 2013. Retrieved 26 July 2011.
- ^ "About Us". The Thalidomide Trust. Retrieved 21 April 2020.
- ^ Scott C, Haupt O (3 May 2015). "The forgotten victims". The Sunday Times Magazine. pp. 12–19. Archived from the original on May 27, 2015. Retrieved 8 May 2015.
- ^ a b Swan, Norman (28 June 2018). "Dr William McBride: The Flawed Character Credited with Linking Thalidomide to Birth Defects." ABC.net.au. Retrieved 29 May 2019.
- ^ Anon. "Widukind Lenz". who named it?. Ole Daniel Enersen. Retrieved 1 May 2009.
- ^ a b Anon (7 June 2002). "Thalidomide:40 years on". BBC News. BBC. Retrieved 1 May 2009.
- ^ "Report of Thalidomide at University of New South Wales". Embryology.med.unsw.edu.au. Archived from the original on 28 October 2012. Retrieved 30 December 2012.
- ^ Millikin, Robert (20 February 1993). "'Thalidomide Doctor' Guilty of Medical Fraud: William McBride, Who Exposed the Danger of One Anti-Nausea Drug, Has Been Disgraced by Experiments with Another." The Independent. Retrieved 28 May 2019.
- ^ Somers GF (1963). "The foetal toxicity of thalidomide". Proc. European Soc. Study Drug Toxicity. 1: 49.
- ^ King C, Kendrick F (1962). "Teratogenic effects of thalidomide in the Sprague Dawley rat". The Lancet. 280 (7265): 409–17. doi:10.1016/S0140-6736(62)90822-X. PMID 14288814.
- ^ McColl JD, Globus M, Robinson S (1965). "Effect of some therapeutic agents on the developing rat fetus". Toxicology and Applied Pharmacology. 7 (7265): 409–17. doi:10.1016/S0140-6736(62)90822-X. PMID 14288814.
- ^ Botting J (April 2015). "Chapter 18". Animals and Medicine: The Contribution of Animal Experiments to the Control of Disease. OpenBook Publishers. ISBN 9781783741175. Retrieved 12 August 2015.
- ^ "Australian thalidomide victims win right for hearing". ABC News. 19 December 2011.
- ^ Petrie A (19 July 2012). "Landmark thalidomide payout offers hope for thousands". The Sydney Morning Herald. Retrieved 14 February 2017.
- ^ Farnsworth S (7 February 2014). "Supreme Court formally approves $89m compensation payout for Thalidomide victims". Australian Broadcasting Corporation. Retrieved 14 February 2017.
- ^ Australian Associated Press (7 February 2014). "Thalidomide survivors' compensation approved". The Sunday Star-Times. Retrieved 14 February 2017.
- ^ "DDR-Bürger schliefen ohne Contergan" [East German citizens slept without thalidomide]. Neues Deutschland (in German). 4 November 2007. Retrieved June 6, 2013.
- ^ a b c d e Thomann KD (2007). "Die Contergan-Katastrophe: Die trügerische Sicherheit der "harten" Daten" [The thalidomide disaster: The false security of 'hard' data]. Deutsches Ärzteblatt (in German). 104 (41): A–2778 / B–2454 / C–2382. Archived from the original on July 28, 2012. Retrieved June 6, 2013. Alt URL Archived 2013-10-19 at the Wayback Machine
- ^ a b Zichner L, Rauschmann MA, Thomann KD (2005). Die Contergankatastrophe eine Bilanz nach 40 Jahren [The thalidomide catastrophe takes stock after 40 years] (in German). Darmstadt: Steinkopff. ISBN 978-3-7985-1479-9.
- ^ a b Webb JF (November 1963). "Canadian Thalidomide Experience". Canadian Medical Association Journal. 89 (19): 987–92. PMC 1921912. PMID 14076167.
- ^ "Turning Points of History–Prescription for Disaster". History Television. Archived from the original on September 29, 2011. Retrieved 24 February 2010.
- ^ a b Racz WJ, Ecobichon DJ, Baril M (August 2003). "On-line sources of toxicological information in Canada". Toxicology. 190 (1–2): 3–14. Bibcode:2003Toxgy.190....3R. doi:10.1016/S0300-483X(03)00192-6. PMID 12909394.
- ^ "The plight of the thalidomide 'sample babies' who don't qualify for gov't compensation". W5. Retrieved 28 November 2016.
- ^ Warren R (2001). "Living in a world with thalidomide: a dose of reality". FDA Consumer. 35 (2): 40. PMID 11444250.
- ^ "TVAC and its mission – Thalidomide". June 24, 2009. Archived from the original on 2009-06-24.
- ^ a b Franks ME, Macpherson GR, Figg WD (May 2004). "Thalidomide". Lancet. 363 (9423): 1802–11. doi:10.1016/S0140-6736(04)16308-3. PMID 15172781. S2CID 208789946.
- ^ a b Braun W (29 December 2015). "Thalidomide: The Connection Between a Statue in Trafalgar Square, a 1960s Children's Show Host and the Abortion Debate". Huffington Post. Retrieved 2 December 2017.
- ^ Mekdeci B. "Bendectin Part 1: How a Commonly Used Drug Caused Birth Defects". Archived from the original on 18 December 2013.
- ^ "Click – Debating Reproductive Rights – Reproductive Rights and Feminism, History of Abortion Battle, History of Abortion Debate, Roe v. Wade and Feminists". www.cliohistory.org. Retrieved 2 December 2017.
- ^ Harris G (13 September 2010). "The Public's Quiet Savior From Harmful Medicines". The New York Times.
- ^ "Dr. Helen Brooke Taussig". U.S. National Library of Medicine. Bethesda, MD. 3 June 2015.
- ^ "10.000 Fälle von Missbildungen" [10,000 cases of malformations] (in German). ORF. Retrieved July 5, 2023.
- ^ "Speech on the occasion of the inauguration of Thalidomide-Memorial". Grünenthal GmbH Website. Archived from the original on 1 September 2012.
- ^ "Thalidomide apology insulting, campaigners say". BBC News. September 1, 2012. Archived from the original on 16 March 2016.
- ^ Jose, Renju (2023-11-13). "Australia to apologise half a century after 'Thalidomide tragedy'". Reuters. Retrieved 2023-11-13.
- ^ "Australian government will apologise to people affected by thalidomide tragedy | Health | The Guardian". amp.theguardian.com. Retrieved 2023-11-13.
- ^ "Outstanding eight to receive honorary doctorates at Convocation". Daily News. Windsor, Ontario, Canada: University of Windsor. 9 June 2016. Retrieved 6 March 2017.
- ^ Mitchell D, Snyder S (June 2005). "Exploitations of embodiment: Born Freak and the academic bally plank". Disability Studies Quarterly. 25 (3). doi:10.18061/dsq.v25i3.575.
- ^ "NoBody's Perfect (2008): Release Info". IMDB. Retrieved 6 June 2013.
- ^ Brussat F, Brussat MA. "Film Review: NoBody's Perfect". Spirituality & Practice. Retrieved 6 June 2013.
- ^ Tamplin H (12 June 2015). "Mid Sussex residents honoured by Queen". Mid Sussex Times. Retrieved 27 December 2015.
- ^ "Orpheus lives: A small good thing in Quastoff". The Portland Phoenix. April 19, 2002. Archived from the original on 6 March 2012. Retrieved 6 June 2013.
- ^ "50 Years: The Kefauver-Harris Amendments". Food and Drug Administration (United States). Retrieved 6 June 2013.
- ^ "Thalidomide". National Health Service (England). Archived from the original on 3 December 2013. Retrieved 6 June 2013.
- ^ Conroy S, McIntyre J, Choonara I (March 1999). "Unlicensed and off label drug use in neonates". Archives of Disease in Childhood: Fetal and Neonatal Edition. 80 (2): F142–4, discussion F144–5. doi:10.1136/fn.80.2.F142. PMC 1720896. PMID 10325794.
- ^ "The evolution of pharmacy, Theme E, Level 3 Thalidomide and its aftermath" (PDF). Royal Pharmaceutical Society. 2011. Archived from the original (PDF) on 14 October 2011.
Further reading
[edit]- Stephens T, Brynner R (2001-12-24). Dark Remedy: The Impact of Thalidomide and Its Revival as a Vital Medicine. Perseus Books. ISBN 978-0-7382-0590-8.
- Knightley P, Evans H (1979). Suffer The Children: The Story of Thalidomide. New York: The Viking Press. ISBN 978-0-670-68114-3.
External links
[edit]- Congenital amputations
- Drug safety
- 20th-century health disasters
- Health disasters in the United Kingdom
- Leprosy
- Medical controversies
- Medical controversies in the United Kingdom
- Medical controversies in Germany
- Medical controversies in Austria
- Medical controversies in Australia
- Medical controversies in New Zealand
- Medical controversies in the United States
- Medical controversies in Canada
- Medical scandals
- History of disability
