Talk:Nonmetal/Archive 2
| This is an archive of past discussions about Nonmetal. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 | Archive 2 | Archive 3 | Archive 4 | Archive 5 |
Color and state of matter
DePiep raises an interesting point.
Obviously all metals are shiny and all bar Hg are solid. I know a little about the shininess; visible light gets scattered by the delocalised surface electrons.
The nonmetals are shiny (B, Si, Ge, As, Sb, Te; C, black P, Se, I), colored (S, F, Cl, Br) or colorless (H, N, O, and the noble gases).
The mechanism of shininess for the semimetals C, As and Sb is the same as that for the metals.
The mechanism for the rest of the shiny nonmetals, which are all semiconductors, arises from their band gaps being less than equal to the visible spectrum cut off of 1.8 eV. S has a bigger band gap, giving rise to its yellow color.
The mechanism for the remaining colored and colorless nonmetals, has to do with the permitted energy levels that their individual electrons occupy.
Why metals are nearly all solid whereas nonmetals cover all three bases is something I'll need to look further into. I do know that Hg is liquid on account of relativistic effects.
Par for the course, there is a little bit of overlap going on here in that C, As, Sb have the electronic structure of semimetals, as does Bi. Yet the chemistry of C, As, Sb is largely nonmetallic whereas Bi is regarded as having just enough metallic character to merit being admitted to the metal club. Frex, nitric acid gives carbon dioxide with C, arsenic acid with As, antimony trioxide with Sb, and bismuth nitrate with Bi.
I intend doing this work as a stand alone exercise from John's copyediting, and will post the results here. Sandbh (talk) 05:18, 2 October 2022 (UTC)
- TL;DR. If this is the missing encyclopedic link, it should be in the article. Then, I don't think it has the right proportions. As talkpage post, it doesn't solve the question. DePiep (talk) 06:50, 7 October 2022 (UTC)
- Well in my favourite counterexample to that, sulfuric acid already gives the sulfate with Sb as well as Bi (but not As). To some extent it can be national-traditional variation: from what I remember, Russian authors are likelier to call Sb a metal than English authors (though this may be because Russian authors often don't recognise a "metalloid" category).
- Metals are nearly all solid because the metallic bond is strong and, crucially, extends over the whole network. You really need special circumstances to have a liquid metal: few electrons to share (Rb, Cs, Fr), relativistic pseudo-closed shell configurations (Hg, maybe Cn and Fl), or some weird molecular-like thing going on (Ga). (And here I am assuming 40°C weather to define "liquid".) When the covalent bonds extend over the whole network, we always get a solid, too (B, C, black P, Si, Ge, Se, Te), and sometimes we even get one where it doesn't when van der Waals forces are strong enough (the big ones: white P, S, yellow As, red Se, I). So it's not a metal vs nonmetal thing; it's closer to a giant-structure vs molecular thing (blurring as the molecule gets heavier). Double sharp (talk) 22:36, 9 October 2022 (UTC)
Antimony does not really give a sulfate. Solid antimony sulfate contains infinite ladders of SO4 tetrahedra and SbO3 pyramids sharing corners. It is often described as a mixed oxide, Sb2O3.3SO3. Sandbh (talk) 03:00, 11 October 2022 (UTC)
- If this is your objection to Double sharp's post, that means the rest is a good replacement for the color & phase topic in the article, starters. IOW, this is the encyclopedic approach (some ce todo allright). It also leads to the conclusion that it is not 1st-sentence-worthy. DePiep (talk) 08:19, 12 October 2022 (UTC)
- This is a bad draft of the colour paragraph:
- "Nonmetallic elements are either shiny, colored, or colorless. For graphitic carbon, black phosphorus, arsenic, antimony, selenium and iodine their structures feature varying degrees of delocalised electrons that scatter incoming visible light, resulting in a shiny appearance (Wiberg 2001, p. 416). The colored nonmetals (sulfur, fluorine, chlorine, bromine) absorb some colours (wavelengths) and transmit the complementary colours. For chlorine, its "familiar yellow-green colour...is due to a broad region of absorption in the violet and blue regions of the spectrum" (Elliot 1929, p. 629).^ For the colorless nonmetals (hydrogen, nitrogen, oxygen, and the noble gases) their electrons are held sufficiently strongly such that no absorption occurs in the visible region of the spectrum, and all visible light is transmitted.”
- This is a bad draft of the colour paragraph:
- ^ The absorbed light may be converted to heat or re-emitted in all directions so that the emission spectrum is thousands of times weaker than the incident light radiation.
- Elliot A 1929, "The absorption band spectrum of chlorine", Proceedings of the Royal Society A, vol. 123, no. 792, pp. 629–644
- Wiberg N 2001, Inorganic Chemistry, Academic Press, San Diego. Wiberg is here referring specifically to iodine.
- The difference in solid, liquid or gaseous forms of nonmetals is addressed in the Physical properties section, i.e. "The internal structures and bonding arrangements of the nonmetals explain their differences in form...".
@Double sharp: I've added a paragraph to the Physical section on the colours of nonmetallic elements. Could you please check to see if that looks OK? (The accompanying footnote needs a citation but the rest should be OK.) Thank you, Sandbh (talk) 12:33, 23 October 2022 (UTC)
- It looks pretty much alright to me, based on what I could find in the literature. Double sharp (talk) 19:42, 29 October 2022 (UTC)
RfC on the Classification of elements
Over at WT:ELEMENTS an RfC is opened on the topic highly relevant on the nonmetals too. See § Request for comment on the classification of chemical elements. You are invited to participate. DePiep (talk) 07:23, 17 February 2023 (UTC)
Article could be revised and renamed: Main Group Chemistry
The article is nice, although the topic called Main Group chemistry might be more appropriate. Has that idea been discussed? --Smokefoot (talk) 22:25, 11 April 2023 (UTC)
- Ealier discussion: WT:CHEM § Nonmetal: Help with copyediting?. Could be that Main Group is a valid topic, but I do not see how it would be an alternative for this topic. That is, replacing nonmetal chemistry. (Incidentally, does it differ from main group?) DePiep (talk) 06:29, 12 April 2023 (UTC)
- Good point. You are correct: Nonmetal is a thing. The article is essential. But the chemistry stuff (reactions, structures, minerals) should mostl be in Main Group Chemistry.
- Yes, I realize that I am discussing this issue in two places, but I dont think that anyone at the Chem Project cares.--Smokefoot (talk) 20:08, 12 April 2023 (UTC)
- No problem, Smokefoot, I only added the link to keep the arguments, already made, at hand. I assume this talkpage is best place. DePiep (talk) 06:19, 13 April 2023 (UTC)
Nonmetal halogen, halogen nonmetal
Both terms are found in the literature.
Since there are alkali metals, alkaline earth metals, and transition metals, i.e. the convention is to put "metals" last, I've replaced mentions of "nonmetal halogens" with "halogen nonmetals". --- Sandbh (talk) 08:24, 24 April 2023 (UTC)
Astatine
Isn't astatine a metalloid? Here it says astatine is a metal, but most other sources say it's a metalloid or nonmetal? 2603:6000:8740:54B1:21BE:C597:B635:6023 (talk) 17:33, 26 February 2023 (UTC)
- In lists of metalloids, astatine appears about 40% of the time.
- The bulk properties of astatine remain unknown as a visible quantity of it would immediately self-vaporize from the heat generated by its radioactivity. It remains to be seen if, with sufficient cooling, a macroscopic quantity could be deposited as a thin film. Historically, "since elements in heavier periods often resemble their n+1 and n−1 neighbours more than their lighter congeners, astatine…was expected to be radioactive and metallic like polonium."[1]
- Qualitative and quantitative assessments of its status, including having regard to relativistic effects, have been consistent with it being a metal:
- 1940. Astatine was judged to be a metal when it was first synthesized.[2] That assessment was consistent with some metallic character seen in iodine,[3] its lighter halogen congener.
- 1972. Batsanov calculated astatine would have a band gap of 0.7 eV[4] (but see the 2013 entry)
- 1983. Edwards and Sienko speculated that, on the basis of the non-relativistic Goldhammer-Herzfeld criterion for metallicity, astatine was probably a metalloid.[5] As the ratio is based on classical arguments[6] it does not accommodate the finding that polonium (cf. 2006 entry following) adopts a metallic (rather than covalent) crystalline structure, on relativistic grounds.[7] Even so it offers a first order rationalization for the occurrence of metallic character amongst the elements.[8]
- 2002. Siekierski and Burgess presumed astatine would be a metal in the context of some of the properties of iodine.[9]
- 2006. Restrepo et al., on the basis of a comparative study of 128 known and interpolated physiochemical, geochemical and chemical properties of 72 of the elements, reported that astatine appeared to share more in common with polonium (a metal) than it did with the established halogens and that, “At should not be considered as a halogen."[10] In so doing they echoed the 1940 observation that, "The chemical properties of the unknown substance are very close to those of polonium."[11]
- 2010. Thornton and Burdette observed that "Since elements in heavier periods often resemble their n+1 and n-1 neighbours more than their lighter congeners, eka-iodine [astatine]...was expected to be radioactive and metallic like polonium." [12]
- 2013. Hermann, Hoffmann, and Ashcroft predicted At would be an fcc metal, once all relativistic effects are taken into account, and that it would have a band gap of 0.68 eV (cf. Batsanov) if only some of these effects were taken into account.[13]
- While astatine could reasonably be presumed to be a metalloid based on ordinary periodic trends, relativistic effects—as seen in gold, mercury, and the heavier p-block elements—are expected to result in condensed astatine being a ductile FCC metal. It could also be expected to show significant nonmetallic character, as is normally the case for metals in, or in the vicinity of, the p-block.
- The suggested distinguishing criteria for metals and nonmetals place At in a metal quadrant.
- References
- 1. Thornton BF & Burdette SC 2010, “Finding eka-iodine: Discovery priority in modern times”, Bulletin for the History of Chemistry, vol. 25, no. 2, pp. 86−96
- 2. Vasáros L & Berei K 1985, General properties of astatine, in Kugler HK & Keller C (eds), Gmelin Handbook of Inorganic and Organometallic chemistry, 8th ed., At, Astatine, system no. 8a, Springer-Verlag, pp. 107–28 (109)
- 3. Moody B 1991, Comparative Inorganic Chemistry, 3rd ed., Edward Arnold, London, p. 303
- 4. Batsanov SS 1971, Quantitative characteristics of bond metallicity in crystals, Journal of Structural Chemistry, vol. 12, no. 5, pp. 809–813 (811).
- 5. Edwards PP & Sienko MJ 1983, On the occurrence of metallic character in the Periodic Table of the Elements, Journal of Chemical Education, vol. 60, no. 9, p. 692
- 6. Edwards PP 1999, Chemically engineering the metallic, insulating and supercon-ducting state of matter, in Seddon KR & Zaworotko M (eds), Crystal Engineering: The Design and Application of Functional Solids, Kluwer Academic, Dordrecht, p. 416
- 7. Encyclopedia of the Structure of Materials, Elsevier, Oxford, p. 142; Pyykkö P 2012, Relativistic effects in chemistry: More common than you thought, Annual Review of Physical Chemistry, vol. 63, p. 56
- 8. Edwards PP & Sienko MJ 1983, On the occurrence of metallic character in the Periodic Table of the Elements, Journal of Chemical Education, vol. 60, no. 9, p. 695
- 9. Siekierski S & Burgess J 2002, Concise Chemistry of the Elements, Horwood Press, Chichester, p. 122
- 10. Restrepo G, Llanos EJ & Mesa H, Topological space of the chemical elements and its properties, Journal of Mathematical Chemistry, vol. 39, p. 411
- 11. Corson DR, MacKenzie R & Segrè E 1940, Possible production of radioactive isotopes of element 85, Physical Review, vol. 57, p. 459
- 12. Thornton BF & Burdette SC 2010, Finding eka-iodine: Discovery priority in modern times, Bulletin for the History of Chemistry, vol. 35, no. 2, p. 86
- 13. Hermann A, Hoffmann R & Ashcroft NW 2013, Condensed Astatine: Monatomic and metallic, Physical Review Letters, vol. 111
- — Preceding unsigned comment added by Sandbh (talk • contribs) 03:22, 25 April 2023 (UTC) — Preceding unsigned comment added by Dhtwiki (talk • contribs)
- @Dhtwiki and Sandbh: To deconfuse. Currently this article makes this top level distinction: Metal-Nonmetal only. So, no major class Metalloids in there. The Metalloids appear as subdivision of class Nonmetals. The article is rewritten this way by Sandbh, with the pre-overhaul GA-icon kept undiscussed.
- So the OP
Isn't astatine a metalloid?
(i.e., not a Metal), in this class scheme requires redefinition of At as a Nonmetal first before it can be subclassified Metalloid. Recent complication: same author (Sandbh) appears to have changed the article object (ie, everything) as "chemically" only [1] while not changing anything to article body, title, TOC or setup???. This is disputable, in various ways, and is disputed. DePiep (talk) 08:41, 25 April 2023 (UTC)
Thank you DePiep.
Yes, the two great classes are (i) metals and (ii) nonmetals. This is a universal distinction. "Metalloids" is only a sometimes classification.
The treatment of elements occupying the frontier territory where the metals meet the nonmetals varies from author to author. Some consider them separate from both metals and nonmetals (and refer to them as metalloids); some regard them as nonmetals or as a sub-class of nonmetals. Other authors count some of them as metals, for example arsenic and antimony, due to their similarities to heavy metals. It has been known for over 100 years that the elements commonly recognised as metalloids (B, Si, Ge, As, Sb, Te) behave chemically like nonmetals. The article treats them as "metalloid nonmetals" in light of their chemical behavior, and for comparative purposes. The metalloids further meet the criteria for nonmetals of low density and relatively high electronegativity.
Astatine has at various times been counted as a metal, metalloid, or nonmetal.
It isn't included in the article since it has been counted as a (post-transition) metal. The article says:
- "Astatine, the fifth halogen, is often ignored on account of its rarity and intense radioactivity;[17] theory and experimental evidence suggest it is a metal."[18]
The edit in question changed the opening sentence from:
- "In chemistry, a nonmetal (or non-metal) is a chemical element..."
- TO
- "A nonmetal (or non-metal) is a chemical element..."
There was no change to the article object, since nonmetals are still referred to in terms of physical and chemical properties. Sandbh (talk) 06:39, 27 April 2023 (UTC)
Hatnote "In chemistry"
Added hatnoting "This article is about nonmetal elements in chemistry" [2] is confusing, sloppy and not correct.
For starters, the body and the TOC say otherwise (eg "Physics"); this distinction is not made. Further hatnote specs (astronomy, metallicity, nonmetallic substances, in physics, valence and conduction bands) add to the confusion/mistake. If article content is changed into this, the article should be moved. But morte likely this is inappropriate application of {{hatnote}}; more like trying to fit topic description (lede issue) in a WP:HATNOTE.
I request and expect Sandbh starts a talk on this page proposing all desired changes coherently. DePiep (talk) 06:27, 24 April 2023 (UTC)
- Thanks DePiep for your interest. I followed WP:HAT in adding the hatnote.
- The only other mention of "physics" is in the Discovery section of the main body of the article, which says:
- "Chemistry- or physics-based techniques used in the isolation efforts were spectroscopy, fractional distillation, radiation detection, electrolysis, ore acidification, displacement reactions, combustion and heating; a few nonmetals occurred naturally as free elements."
- This does not have anything do with the concept of a nonmetal in physics.
- Astronomy and materials science are not mentioned in the main body.
--- Sandbh (talk) 07:14, 24 April 2023 (UTC)
- Name change I'm inclined to change the name of the article to "Nonmetal (chemistry)" and to create disambiguation links for:
- Nonmetal (astronomy) --> Metallicity
- Nonmetal (physics) --> Valence and conduction bands
- The hatnote would be reduced to "For nonmetallic substances see Materials science."
- Sandbh (talk) 01:13, 28 April 2023 (UTC)
I've renamed the article to Nonmetal (chemistry) and trimmed the hatnote, after setting up redirects for Nonmetal (astronomy) and Nonmetal (physics). --- Sandbh (talk) 07:26, 30 April 2023 (UTC)
List of miscellaneous items
So, taking a dive in this article, specifically this version:
- "largely make up the" I know this is shortest, but would "make up most of the" be a bit sounder grammatically? Done
- "namely boron; silicon and germanium; arsenic and antimony; and tellurium" might work better as a parenthetical. Done
- Footnote 6 I think "both metals" is better than "metals both" Done
- I know, hypocritical from me but the "Physical" section could benefit from less instances of "occurs" Done
- "as used in non-stick coatings for pans and other cookware." is unreferenced. Done
- "From right to left in periodic table terms, three or four kinds of nonmetals are more or less commonly discerned. These are: the relatively inert noble gases;" are also unreferenced. Done
- "Metalloids are here treated as nonmetals in light of their chemical behavior, and for comparative purposes." is unreferenced. Done
- "In 2014 it was reported that the Earth's core" sounds a bit like a WP:PROSELINE thing; can it be reworded to be less time-dependent? Done
- "Dingle explains the situation this way:" who is this Dingle? Done
- "Oxygen is found in the atmosphere; in the oceans as a component of water; and in the crust as oxide minerals." lacks a reference. Done
- I don't think that having a "daily cost" item is a good idea. I doubt that such prices are stable enough that they could be kept up-to-date with reasonable effort.
- I rechecked the costs as at April 2023 since I did this originally as at August 2022. In the ensuing eight months there was hardly any variation, in relative terms. I suspect that an annual check would suffice, an idea I got from you. Sandbh (talk) 08:20, 3 May 2023 (UTC)
- "Chemistry- or physics-based techniques used in the isolation efforts were spectroscopy, fractional distillation, radiation detection, electrolysis, ore acidification, displacement reactions, combustion, and heating; a few nonmetals occurred naturally as free elements." lacks a reference. Done
- "Physical properties apply to elements in their most stable forms under ambient conditions, and are listed in loose order of ease of determination. Chemical properties are listed from general to descriptive, and then to specific. The dashed line around the metalloids denotes that, depending on the author, the elements involved may or may not be recognized as a distinct class or subclass of elements. Metals are included as a reference point." lacks a reference. Done
- What is the source for the ionization energies in the comparison table? Done
- Footnotes 4, 12, 13, 26 need a source Not applicable
- Note 4 does not need a cite since it is only listing the grayed-out elements in the parent image; ditto note 12 sets out the first row elements in the parent image; note 13 as per note 4; note 26 is a meta-explanation of what is going on in the sentence.
- Footnote 14 lacks a reference for one sentence Not applicable
- That is an introductory and explanatory sentence; the cites are in the following three sentences/paras.
- Footnote 23 has the somewhat mysterious "combined with sulfur" Done
I can do a source spot-check if so desired. Jo-Jo Eumerus (talk) 09:30, 2 May 2023 (UTC)
- Tx JJ for this impressive list. I count 17 items, nine of which are for missing references. More refs should be no problem. The remaining eight items should be OK to address.
- A source spot check was completed in one of the more recent FAC nominations by, as I recall, Complex rational.
- --- Sandbh (talk) 07:35, 3 May 2023 (UTC)
- All items have now been addressed. Sandbh (talk) 13:32, 3 May 2023 (UTC)
First paragraph of lede
The first paragraph of the lede starts by explaining what nonmetals are not:
- "A nonmetal (or non-metal) is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). They are usually poor conductors of heat and electricity, and brittle or crumbly when solid, due to their electrons having low mobility. In contrast, metals are good conductors and most are easily flattened into sheets and drawn into wires since their electrons are generally free-moving. Nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds."
I am thinking about changing this so that nonmetals are instead explained in terms of what they are:
- "A nonmetal is a type of chemical element that is a poor electrical conductor or is a mechanically weak and brittle solid the most stable oxide of which is acidic. They range from colorless gases (like hydrogen) to shiny substances (like carbon, as graphite). Their electrons have low mobility. In contrast, metals are good conductors and most are easily flattened into sheets and drawn into wires since their electrons are generally free-moving. Nonmetal atoms tend to attract electrons in chemical reactions and and to form acidic compounds."
The reference to poor electrical conductivity applies to nearly all nonmetals. Carbon, as graphite, is an exception. But it is a mechanically weak and brittle substance, and CO2 is an acidic oxide.
Among the metals, and semimetals (in a physics-based sense), gallium, arsenic, antimony and bismuth are brittle and mechanically weak. Gallium trioxide is amphoteric; aqueous solutions of arsenic trioxide are weakly acidic; antimony trioxide is amphoteric, but has acidic properties predominating; and bismuth trioxide is basic.
--- Sandbh (talk) 02:23, 28 April 2023 (UTC)
- Well, how do the sources typically define things?
- It seems to me that nonmetallicity is more correlated with keeping one's own electrons than attracting others', i.e. electronegativity rather than electron affinity. The noble gases are a clear example. Also, Cs has a higher electron affinity than B. Electronegativity is also not perfect considering that Au beats Si by this measure, but at least it doesn't have literally alkali metals beating nonmetals, but the noble metals. Double sharp (talk) 10:41, 28 April 2023 (UTC)
The sources are about 50:50 between defining nonmetals as (i) elements either not having the properties of a metal or (ii) in terms of more specific properties as per following 20 examples from the literature:
- …a substance that conducts heat and electricity poorly, is brittle or waxy or gaseous, and cannot be hammered into sheets or drawn into wire. Nonmetals gain electrons easily to form anions.
- A nonmetal is an element whose atoms tend to gain (or share) electrons.
- A nonmetal is a chemical element that is mechanically weak in its most stable form, brittle if solid, and usually gains or shares electrons in chemical reactions.
- As a result of the free electron, graphite, though a nonmetal, is able to conduct electricity while diamond cannot.
- The distinctive chemical property of a nonmetal is the ability to gain electrons to form an anion when reacting with a metal. The nonmetals have large ionization energies and most have negative electron affinities.
- If the oxide of a nonmetal is placed in water, the mixture will be acid.
- The simplest way to tell a metal from a nonmetal is that most nonmetals do not conduct thermal energy or electricity.
- nonmetals are insulators, with a few rare exceptions.
- A nonmetal is an element that gains or shares electrons when it combines chemically. There is no set of physical properties that applies to all nonmetals, there is for the metals.
- A metal is a lustrous malleable element that is a good conductor of heat and electricity; a nonmetal is an element that is a poor conductor.
- A nonmetal is an element that tends to gain valence electrons in chemical reactions, becoming an anion in the process.
- Chemically, the property of an element that makes it a nonmetal is the element's ability to gain electrons.
- A nonmetal is an element that usually has a low density, a low melting point, and is a poor conductor of heat and electricity.
- A nonmetal is an element that is relatively easily reduced.
- A nonmetal is a kind of matter that does not have a metallic luster, is a poor conductor of heat and electricity, and when solid, is a brittle material that cannot be pounded or pulled into new shapes.
- A nonmetal is one of a number of elements, including gases, liquids and solids, which are grouped together because they do not conduct heat or electricity well, are not ductile and malleable, and do not reflect light well. Chemically, nonmetal atoms form negative ions.
- Bands in metals In terms of the band theory, the distinction between a metal and a non-metal is that in the former there are incompletely filled bands.
- the principal chemical property of a non-metal is its ability to form a negatively charged anion by accepting electrons from a metal.
- A non-metal is an element which ionizes by electron gain.
- A non-metal is an element that has four or more valence electrons.
The properties include poor conductivity; brittle and mechanically weak if solid; usually low density and mp; large ionization energy; gain or share electrons; usually negative EA; form negative ions; acidic oxides. --- Sandbh (talk) 02:54, 29 April 2023 (UTC)
I've updated the lede paragraph to refer to what nonmetals are, rather than what they are not. Sandbh (talk) 07:22, 30 April 2023 (UTC)
I found a hybrid nonmetal entry by Read J 1965, in JR Newman (ed.), The International Encyclopedia of Science, Thomas Nelson and Sons, London, p. 832:
- "NONMETALS stand apart from METALS in many ways, both physically and chemically, although these two classes of elements merge into one another. Physically, nonmetals do not exhibit luster or polish; they are poor conductors of heat and electricity; if solid, they are often brittle, they are not ductile, and they possess poor tensile strength. At ordinary temperatures some are solid, some liquid, and some gaseous: their melting points range from −272°C(−457.6°F), under 26 atm pressure, for helium, to above 3550°C (6422°F) for carbon. Their values for specific gravity are low, compared with those for metals. Chemically, their oxides usually react with water to form acids, and their chlorides are often decomposed by water."
It is a good effort but not without errors.
That "nonmetals do not exhibit luster or polish" is contradicted by graphitic C, black P, gray Se, and I. That they are, "if solid...often brittle" is not quite true since all solid nonmetals are brittle, unless he had white P in mind which can be cut with a knife however black P, which is brittle, is the most stable form. That they are "poor conductors of heat and electricity" is contradicted by graphitic C. While Read says that "some" are liquid, bromine is the only liquid nonmetal.
--- Sandbh (talk) 02:39, 1 May 2023 (UTC)
I restored the lede paragraph back to saying what nonmetals aren't since there is no agreement as to what they are. Sandbh (talk) 07:49, 1 May 2023 (UTC)
- Perhaps you could say "Nonmetal is a classification of elements. It commonly refers either to <foo> or <fab>"? If there is more than one common definition of nonmetal, mentioning all of them would be better than implying there is only one accepted definition. Jo-Jo Eumerus (talk) 09:58, 1 May 2023 (UTC)
Simplifying the lede
I've adjusted the lede paragraph, and made associated edits later in the article, to make things easier to follow in terms of what is a nonmetal in the broadest sense of the term.
While there is no common rigorous definition of a nonmetal in terms of the precise properties involved, a broad definition based on relatively low density (a physical property) and relatively high electronegativity (chemical) encompasses:
- the 23 elements within the scope of the article; and therefore
- the other varying conceptions of nonmetals and the resulting sets of of nonmetallic elements
Mention of the relatively low density and relatively high EN of nonmetals is set out in Hein M & Arena S 2013, Foundations of College Chemistry, 14th ed., John Wiley & Sons, pp. 226, G-6.
Here are extracts from a dozen sources corroborating the low density of nonmetals (#2 also refers to high EN):
- A nonmetal is an element that usually has a low density
- Unlike metals, solid non-metals are dull, brittle and not malleable. They also tend to be less dense than metals, and have lower melting and boiling points (apart from carbon). With high electronegativity (see here) non-metal elements ...
- Nonmetals include gases , liquids , and solids . They are generally dull instead of shiny , and they do not conduct heat or electricity very well . They cannot be shaped into wires or thin sheets , and they tend to have a low density .
- Nonmetals have a low density .
- Nonmetals are characterized by lack of luster , lack of conductivity , brittleness , and low density .
- Nonmetals appear on the right side of the periodic table a . These elements usually have a low density
- Dull , reflecting light poorly or absorbing strongly Low density
- Have low density
- Nonmetals are usually lighter in weight than metals ,
- Non- metals are generally lighter in weight than metals
- Non - metals are usually lighter than metals
- Most nonmetals have no luster , are soft , are poor conductors , and have a low density.
And a dozen referring to high(er) electronegativity:
- With high electronegativity (see here) non-metal elements ..
- Table 11.5 shows that the relative electronegativity of the nonmetals is high and that of the metals is low.
- The Allred Rochow electronegativities of the nonmetals are larger than 1.8, those of the metals are smaller than 1.5
- Metals, in the lower left corner of the table, have low electronegativities and nonmetals, in the upper right
- These two trends result in nonmetals generally having higher electronegativities than metals
- The electronegativities of metals are small while those of nonmetals are large. These data are useful in the classification of metals from non-metals
- Elements with high electronegativity (such as nonmetals) have a greater ability to attract electrons
- Metals are the least electronegative elements (they are electropositive) and nonmetals the most electronegative.
- Some of the elements have high values of electronegativity and some have lower values. Those with low electronegativity values are called metals and those with high electronegativity values are categorized as nonmetals
- Nonmetals are much more electronegative than metal.
- The most electronegative elements are the nonmetals on the far right of the periodic table
- Nonmetals have high electronegativities.
--- Sandbh (talk) 07:04, 24 May 2023 (UTC)
Merge or split?
It seems that it would be much better if either the history section is split and merged with Discovery of the nonmetals, or the opposite, merge the discovery article here. It is short enough to go both ways. ReyHahn (talk) 14:12, 26 July 2023 (UTC)
Halogen pic
|
How would something like this work for the picture at Nonmetal § Halogen nonmetals? sodium (Na), chlorine (Cl), and table salt (NaCl) Corrosive chlorine, a halogen nonmetal, combines with highly reactive sodium to form stable, unreactive table salt.
|
Sources pic
|
I’m experimenting here with a table to replace the one at § Abundance, extraction, and uses
References Differences from the status quo:
YBG (talk) 15:00, 6 November 2023 (UTC)
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Abundance, sources, uses
|
@Sandbh, @Double sharp: What do you think of putting each graphic in its proper section? Check out special:permalink/1183897347 § Abundance, sources, and uses in both desktop and mobile views. YBG (talk) 04:40, 7 November 2023 (UTC)
It looks good. --- Sandbh (talk) 12:12, 18 November 2023 (UTC) |
Whither now (post FAC7)
|
@Sandbh: Thank you for taking the initiative to resolve the outstanding issues in FAC-7. I wonder if it might be wise to see how many of the reviewers would be willing to become co-nominators? I would be willing to do this under certain circumstances, and I think others might also. Convincing previous reviewers to become co-nominators will improve (but delay) the FAC-8 nomination. For me to be willing to do this, I would need to engage with the FA criteria in a way I have not yet done. For each criterion, I would wish to state the extent to which I reviewed it and list any outstanding issues that need to be addressed before I'm willing to become a co-nominator. Potential problems with this:
Is there any interest in pursuing such a process? Is anyone else willing to consider becoming a co-nominator? YBG (talk) 09:34, 23 October 2023 (UTC)
@YBG: The plan is to not renominate until all the discusssion on this page has been concluded. --- Sandbh (talk) 02:11, 31 October 2023 (UTC)
|
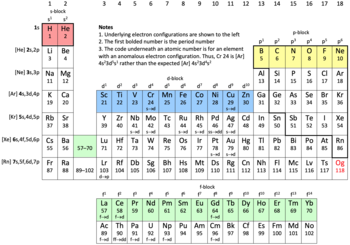
First row anomaly pic
The pic at § First row anomaly has a lot of detail not related to the first row anomaly. This makes the picture cluttered and confusing. I've included here an outline of what could be an alternative. YBG (talk) 13:02, 8 November 2023 (UTC) @YBG: Please proceed, with the exception that the first rows of the d and f blocks do not need to be shaded. --- Sandbh (talk) 12:45, 15 November 2023 (UTC)
 @YBG: They do. The degree to which the anomalies standout is s >> p > d >f. It doesn't really matter if the d- and f- anomalies are included. Perhaps something like attached image. --- Sandbh (talk) 11:31, 18 November 2023 (UTC)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
More re complementary pairs
Metalloid / PTM comparison
|
The penultimate paragraph with block quote amounts to saying “The metalloids and PTM are in the middle of the periodic table, one is weakly metallic, the other weakly nonmetallic”. This is not really a fact about the M-oids and PTM, but rather a fact about the general strongly-metallic-to-strongly-nonmetallic PT trend. The text admits that the comparison is only occasionally made. Unlike the group 17 and 18 comparisons, I don't think this paragraph has anything substantial enough here to be included in the 1st paragraph of the section as I added in the now reverted edits. Best to simply delete this paragraph. YBG (talk) 15:39, 30 October 2023 (UTC)
Done. --- Sandbh (talk) 12:48, 15 November 2023 (UTC) |
Unclassified NM / TM comparison
|
The penultimate “In terms of PT geography...” paragraph essentially says both classes are between more reactive elements and less reactive elements. This is not really a fact about the UNM and TM, but rather a fact about the general strongly-metallic-to-strongly-nonmetallic PT trend. Unlike the group 17 and 18 comparisons, I don't think this paragraph has anything substantial enough here to be included in the 1st paragraph of the section as I added in the now reverted edits. Best to simply delete this paragraph. YBG (talk) 15:39, 30 October 2023 (UTC)
Done. --- Sandbh (talk) 12:48, 15 November 2023 (UTC) |
Comparisons in general
|
If you are willing to delete all four comparison paragraphs, I would entertain the addition at the end of the introduction to Types, a general statement describing NM L-R trend, mentioning that it mostly mirrors the L-R trend in metals. If this seems a good idea, let me know and once all four comparison paragraphs are removed, I'll add it from my offline draft. YBG (talk) 15:39, 30 October 2023 (UTC)
Sandbh’s analysis of YBG’s alternative
Discussion of YBG’s alternative@Sandbh: Your longish post seems to me to have three main objections, which I would like to discuss individually. I would appreciate your effort to keep your responses brief. YBG (talk) 21:41, 17 November 2023 (UTC) (1) You object to
(2) You object to
(3) You object to how my paragraph fits into the section flow. I need to think about this more. Let’s wait until after we discuss the other two issues. YBG (talk) 21:41, 17 November 2023 (UTC)
@YBG: It’s not clear to me why there is so much ado over a single paragraph that takes up ca. 1% of the article’s size. I’m currently time-challenged and hope to be able to add some further comments later on. Sandbh (talk) 21:59, 23 November 2023 (UTC) The citation is to Parish's book, The Metallic Elements. He surveys the s-block metals; the f-block metals (a chapter each on Ln and An); the d-block metals (a chapter each on the 3d metals, and the 4d-5d metals (including the noble metals); and the p-block metals. The relevant paragraph in the nonmetal article is accompanied by a table showing EN ranges for the elements. The pattern of electronegativity is plain to see. For the types of nonmetals, there is a progression from less electronegative to more electronegative. A similar progression occurs among the metals. Metallicity is broadly related to EN and to reactivity. So, the s- and f-block metals are the most EN/metallic, the ordinary TM are next, then follow the p-block metals, and the noble metals. Among the metals a similar pattern is seen in the melting point v EN chart in the post-transition metals article. Sandbh (talk) 23:54, 23 November 2023 (UTC)
I may as well gently add, once again, it is well known that both metals and nonmetals range from highly to less reactive (even noble). The paragraph under discussion says just that. Sandbh (talk) 01:25, 24 November 2023 (UTC) |
Further comments (because at the FAC7 I stopped at Physical properties)
Double sharp (talk) 07:35, 30 October 2023 (UTC)
--- Sandbh (talk) 01:11, 16 November 2023 (UTC)
|