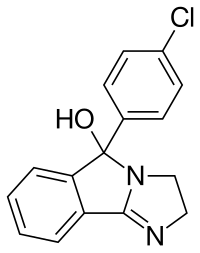
Mazindol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mazanor, Sanorex |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 93% |
| Metabolism | Hepatic |
| Elimination half-life | 10–13 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.764 |
| Chemical and physical data | |
| Formula | C16H13ClN2O |
| Molar mass | 284.74 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Mazindol, sold under the brand names Mazanor and Sanorex, is a central nervous system (CNS) stimulant which is used as an appetite suppressant.[2] It was developed by Sandoz-Wander in the 1960s.[3] The US Food and Drug Administration approved mazindol in June 1973, but Novartis, the manufacturer, discontinued it in 1999 for reasons unrelated to its efficacy or safety.[4]
Medical uses
[edit]Mazindol is used in short-term (i.e., a few weeks) treatment of obesity, in combination with a regimen of weight reduction based on caloric restriction, exercise, and behavior modification in people with a body mass index greater than 30, or in those with a body mass index greater than 27 in the presence of risk factors such as hypertension, diabetes, or hyperlipidemia. Mazindol is not currently available as a commercially marketed and FDA-regulated prescription agent for the treatment of obesity.
Off-label use of mazindol has demonstrated efficacy in treating symptoms of narcolepsy and cataplexy.[5] Studies beginning in the 1970s indicated that mazindol reduced sleep attacks and cataplexy with comparable efficacy to amphetamine, but with reduced cardiovascular side effects.[5][6][7] In 2021, mazindol was identified as an orexin-2 receptor (OX2R) agonist, providing a mechanistic explanation for its therapeutic action in narcolepsy, a condition often linked to orexin system dysfunction. This discovery has prompted further research interest, including the development of modified-release formulations and clinical trials such as the POLARIS program and phase 3 AMAZE trials.[5][8] Preclinical studies have also suggested potential neuroprotective effects in rat models of narcolepsy.[5]
There is a Swiss study investigating its efficacy in treating attention deficit hyperactivity disorder (ADHD).[9]
Additional patented uses include for the treatment of schizophrenia,[10] reducing cravings for cocaine,[11] and for the treatment of neurobehavioral disorders.[12]
Pharmacology
[edit]| Site | Ki (nM) |
|---|---|
| DAT | 25.9 |
| NET | 2.88 |
| SERT | 272 |
Mazindol is a sympathomimetic amine, which is similar to amphetamine. It stimulates the central nervous system, which increases heart rate and blood pressure, and decreases appetite. Sympathomimetic anoretics (appetite suppressants) are used in the short-term treatment of obesity. Their appetite-reducing effect tends to decrease after a few weeks of treatment. Because of this, these medicines are useful only during the first few weeks of a weight-loss program.
Although the mechanism of action of the sympathomimetics in the treatment of obesity is not fully known, these medications have pharmacological effects similar to those of amphetamines. Like other sympathomimetic appetite suppressants, mazindol is thought to act as a reuptake inhibitor of norepinephrine, dopamine, and serotonin. The recommended dosage is 2 mg per day for 90 days in patients 40 kg overweight and under; 4 mg a day in patients more than 50 kg overweight; divided into two doses separated by a 12-hour window between each dose.
Overdose
[edit]Symptoms of a mazindol overdose include: restlessness, tremor, rapid breathing, confusion, hallucinations, panic, aggression, nausea, vomiting, diarrhea,irregular heartbeat, and seizures.
Analogues
[edit]An analogue of mazindol was reported that was stated to be less toxic than the parent drug from which it was derived.[14] It is made from Chemrat (pindone).

QSAR Dialogue
[edit]
From available QSAR data, the following trends are apparent:[16]
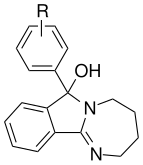
- Desoxylation of the tertiary alcohol in mazindol improves DAT and SERT binding without substantially reducing NET affinity. This compound has been called "mazindane".[17]
- Removal of the p-chlorine atom from the phenyl ring of mazindol increases NET affinity and substantially reduces DAT and SERT affinity.
- Expansion of the imidazoline ring system in mazindol to the corresponding six-membered homolog increases DAT affinity by ~10 fold.
- Replacement of the phenyl moiety with a naphthyl ring system results in a ~50 fold increase in SERT affinity without significant decreases in NET or DAT affinities.
- Halogenation of 3' and/or 4' position of the phenyl ring of mazindol results in increased potency at NET, DAT, and SERT.
- Fluorination of the 7' position of the tricyclic phenyl ring results in a ~2 fold increase in binding affinity to the DAT.
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | R′′ | IC50 (nM) (Inhibition of [3H]WIN 35428 binding) |
IC50 (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|---|---|
| (cocaine) | 89.1 ± 8 | 208 ± 12 | 2.3 | ||||

| |||||||
| (mazindol) | H | H | 4′-Cl | 8.1 ± 1.2 | 8.4 ± 1.3 | 1.0 | |
| 384a | H | H | H | 66.0 ± 8.9 | 124 ± 37 | 1.9 | |
| 384b | H | H | 4′-F | 13.3 ± 1.8 | 25.4 ± 2.7 | 1.9 | |
| 384c | H | 7-F | H | 29.7 ± 7.0 | 78 ± 46 | 2.6 | |
| 384d | H | H | 2′-Cl | 294 ± 6 | 770 ± 159 | 2.6 | |
| 384e | H | H | 3′-Cl | 4.3 ± 0.4 | 9.2 ± 5.3 | 2.1 | |
| 384f | CH3 | H | 4′-Cl | 50.4 ± 5.5 | 106 ± 5.6 | 2.1 | |
| 384g | H | 6-Cl | H | 57.2 ± 8.3 | 58 ± 6.4 | 1.0 | |
| 384h | H | 7-Cl | H | 85.4 ± 14 | 55.17 | 0.6 | |
| 384i | H | 7-F | 4′-Cl | 6.5 ± 1.2 | 15 ± 9 | 2.3 | |
| 384j | H | 7-Cl | 4′-F | 52.8 ± 8.7 | 53 ± 18 | 1.0 | |
| 384k | H | H | 2′,4′-Cl2 | 76.5 ± 1.11 | 92 ± 19 | 1.2 | |
| 384l | H | H | 3′,4′-Cl2 | 2.5 ± 0.5 | 1.4 ± 1.6 | 0.6 | |
| 384m | H | 7,8-Cl2 | 4′-Cl | 13.6 ± 1.5 | |||
| 384n | H | H | 2′-Br | 1340 ± 179 | |||
| 384o | H | H | 4′-Br | 2.6 ± 1.5 | 8.6 ± 3.5 | 3.3 | |
| 384p | H | H | 4′-I | 17.2 ± 0.9 | 14 ± 6.4 | 0.8 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | IC50 (nM) (Inhibition of [3H]WIN 35428 binding) |
IC50 (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|---|

| ||||||
| 388a | H | H | 5.8 ± 1.6 | 18 ± 11 | 3.1 | |
| 388b | H | 2′-F | 23.2 ± 1.7 | 89 ± 2.8 | 3.8 | |
| 388c | H | 3′-F | 2.0 ± 0.02 | 3.1 ± 1.8 | 1.6 | |
| 388d | H | 4′-F | 3.2 ± 1.7 | 8.5 ± 4.9 | 0.4 | |
| 388e | H | 3′-Cl | 1.0 ± 0.2 | 1.3 ± 0.14 | 1.3 | |
| 388f | H | 4′-Cl | 1.7 ± 0.2 | 1.4 ± 0.35 | 0.8 | |
| 388g | CH3 | 4′-Cl | 6.3 ± 4.5 | 1.7 ± 1.6 | 0.3 | |
| ||||||
| 389a | H | 5.9 ± 0.1 | 11 ± 3.2 | 2.0 | ||
| 389b | 4′-Cl | 1.5 ± 0.1 | 3.4 ± 2.3 | 2.3 | ||
| 389c | 3′,4′-Cl2 | 1.7 ± 0.1 | 0.26 ± 0.16 | 0.2 |
| Structure | n | R | R' | R" | hSERT | hNET | hDAT | SERT/DAT Selectivity |
NET/DAT Selectivity |
|---|---|---|---|---|---|---|---|---|---|

| |||||||||
| 1 | Cl | H | OH | 94 ± 32 | 4.9 ± 0.5 | 43 ± 20 | 2.2 | 0.1 | |
| 1 | Cl | H | H | 15 ± 5 | 6.9 ± 1.5 | 6.0 ± 0.7 | 2.5 | 1.2 | |
| 1 | H | H | OH | 2140 ± 450 | 2.8 ± 0.92 | 730 ± 180 | 2.9 | 0.004 | |
| 1 | Naphthyl | OH | 1.8 ± 1.3 | 4.5 ± 1.5 | 66 ± 10 | 0.03 | 0.07 | ||
| 2 | Cl | H | OH | 53 ± 7 | 4.9 ± 0.5 | 3.7 ± 0.4 | 14.3 | 1.3 | |
| 2 | OH | H | OH | 60 ± 19 | 1.9 ± 0.15 | 59.0 ± 3.6 | 1 | 0.03 | |
| 2 | OMe | H | OH | 94 ± 34 | 4.1 ± 1.4 | 30.4 ± 2.4 | 3.1 | 0.1 | |
| 2 | -OCH2O- | OH | 83 ± 29 | 0.62 ± 0.25 | 2.21 ± 0.3 | 37.7 | 0.3 | ||
Chemistry
[edit]Tautomers
[edit]
Mazindol exhibits pH dependent tautomerization between the keto form and the cyclic hemiaminal. Mazindol exists in the tricyclic (-ol) form in neutral media and undergoes protonation to the benzophenone tautomer in acidic media. QSAR studies have indicated that the ability of mazindol to inhibit NE and DA reuptake may be mediated by the protonated (benzophenone) tautomer.[18]
Synthesis
[edit]The precursor for mazindol was described in the synthesis of Chlortalidone.

The synthesis of mazindol starts by reaction of a substituted benzoylbenzoic acid (1) with ethylenediamine. The product 3 can be rationalized as being an aminal from the initially formed monoamide 2. This is then subjected to reduction with LiAlH4 and-without isolation-air oxidation. Reduction probably proceeds to the mixed aminal/carbinolamine 4; such a product would be expected to be in equilibrium with the alternate aminal 5. The latter would be expected to predominate because of the greater stability of aldehyde aminals over the corresponding ketone derivatives. Air oxidation of the tetrahydroimidazole to the imidazoline will then remove 5 from the equilibrium. There is thus obtained the anorectic agent mazindol (6). The synthesis of homomazindol (the six-member ring A homologue) is accomplished by substitution of 1,2-diaminoethane with 1,3-diaminopropane.
An alternative synthesis was described:
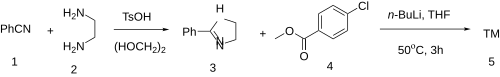
2-Phenyl-2-Imidazoline [936-49-2] (3) Methyl 4-Chlorobenzoate [1126-46-1] (4)
Research
[edit]As of 2016 mazindol was being studied in clinical trials for attention-deficit hyperactivity disorder.[23]
See also
[edit]Notes
[edit]References
[edit]- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Carruba MO, Zambotti F, Vicentini L, Picotti GB, Mantegazza P (1978). "Pharmacology and biochemical profile of a new anorectic drug: mazindol". Cent. Mech. Anorectic Drugs: 145–64.
- ^ a b US granted 3597445, Houlihan WJ, Eberle MK, "1H-Isoindole Intermediates", issued 3 August 1971, assigned to Sandoz AG
- ^ "Determination That SANOREX (Mazindol) Tablets 1 and 2 Milligrams Were Not Withdrawn From Sale for Reasons of Safety or Effectiveness". Federal Register. 15 July 2008. Retrieved 28 December 2024.
- ^ a b c d Konofal E (August 2024). "From past to future: 50 years of pharmacological interventions to treat narcolepsy". Pharmacology, Biochemistry, and Behavior. 241: 173804. doi:10.1016/j.pbb.2024.173804. PMID 38852786.
- ^ Parkes JD, Schachter M (October 1979). "Mazindol in the treatment of narcolepsy". Acta Neurologica Scandinavica. 60 (4): 250–4. doi:10.1111/j.1600-0404.1979.tb02976.x. PMID 525256.
- ^ Alvarez B, Dahlitz M, Grimshaw J, Parkes JD (May 1991). "Mazindol in long-term treatment of narcolepsy". Lancet (London, England). 337 (8752): 1293–4. doi:10.1016/0140-6736(91)92966-6. PMID 1674093.
- ^ Corser B, Stern T, Bogan R, Franco J, Apostol G, Konofal E, Morse A, Rosenberg R, Kushida C, Thorpy M (29 May 2023). "0585 A four-week randomized, double-blind, placebo-controlled, phase 2 study of mazindol ER in the treatment of narcolepsy" (PDF). SLEEP. 46: A257 – A257. doi:10.1093/sleep/zsad077.0585. ISSN 0161-8105. Retrieved 28 December 2024.
- ^ Grover N (2017-05-31). "Swiss biotech NLS Pharma's ADHD drug succeeds in mid-stage study". Reuters. Retrieved 2021-07-15.
- ^ US 5447948, "Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia", issued 5 September 1995, assigned to Yale University
- ^ US 5217987, Berger SP, "Dopamine uptake inhibitors in reducing substance abuse and/or craving", issued 8 June 1993
- ^ WO 2009155139, Kovacs B, Pinegar L, "se of isoindoles for the treatment of neurobehavioral disorders", published 23 December 2009, assigned to Afecta Pharmaceuticals Inc
- ^ Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707. S2CID 15573624.
- ^ Lemke TL, Cates LA, Steenberg M, Cho YM (August 1975). "Analogs of the anorexic mazindol". Journal of Pharmaceutical Sciences. 64 (8): 1375–8. doi:10.1002/jps.2600640824. PMID 1151711.
- ^ a b c Singh S (March 2000). "Chemistry, design, and structure-activity relationship of cocaine antagonists" (PDF). Chemical Reviews. 100 (3): 925–1024. doi:10.1021/cr9700538. PMID 11749256.
- ^ a b c Houlihan WJ, Ahmad UF, Koletar J, Kelly L, Brand L, Kopajtic TA (September 2002). "Benzo- and cyclohexanomazindol analogues as potential inhibitors of the cocaine binding site at the dopamine transporter". Journal of Medicinal Chemistry. 45 (19): 4110–8. doi:10.1021/jm010301z. PMID 12213054.Houlihan WJ, Kelly L, Pankuch J, Koletar J, Brand L, Janowsky A, Kopajtic TA (September 2002). "Mazindol analogues as potential inhibitors of the cocaine binding site at the dopamine transporter". Journal of Medicinal Chemistry. 45 (19): 4097–109. doi:10.1021/jm010302r. PMID 12213053.
- ^ Houlihan WJ, Kelly L (January 2003). "Assessment of mazindane, a pro-drug form of mazindol, in assays used to define cocaine treatment agents". European Journal of Pharmacology. 458 (3): 263–73. doi:10.1016/s0014-2999(02)02791-7. PMID 12504782.
- ^ Koe BK (December 1976). "Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain". The Journal of Pharmacology and Experimental Therapeutics. 199 (3): 649–661. PMID 994022.
- ^ Aeberli P, Eden P, Gogerty JH, Houlihan WJ, Penberthy C (February 1975). "5-aryl-2,3-dihydro-5H-imidazo[2,1-a]isoindol-5-ols. A novel class of anorectic agents". Journal of Medicinal Chemistry. 18 (2): 177–82. doi:10.1021/jm00236a014. PMID 804553.
- ^ DE granted 1814540, Houlihan WJ, "Improvements in or Relating to Imidazoisoindole Derivatives", issued 3 July 1969, assigned to Sandoz AG
- ^ DE granted 1930488, Houlihan WJ, Eberle MK, "Heterocyclische Verbindungen und Verfahren zu ihrer Herstellung", issued 19 March 1970, assigned to Sandoz AG
- ^ US granted 3763178, Sulkowski TS, "Midazolinyl Phenyl Carbonyl Acid Addition Salts and Related Compounds", issued 2 October 1973, assigned to American Home Products
- ^ Mattingly GW, Anderson RH (December 2016). "Optimizing outcomes in ADHD treatment: from clinical targets to novel delivery systems". CNS Spectrums. 21 (S1): 45–59. doi:10.1017/S1092852916000808. PMID 28044946. S2CID 24310209.
