Levopropoxyphene
| |
| Names | |
|---|---|
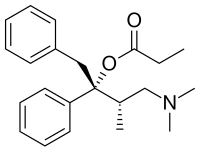
| Preferred IUPAC name
(2R,3S)-4-(Dimethylamino)-3-methyl-1,2-diphenylbutan-2-yl propanoate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C22H29NO2 | |
| Molar mass | 339.471 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Levopropoxyphene is an antitussive. It is an optical isomer of dextropropoxyphene. The racemic mixture is called propoxyphene. Only the dextro-isomer (dextropropoxyphene) has an analgesic effect; the levo-isomer appears to exert only an antitussive effect. It was formerly marketed in the U.S. by Eli Lilly under the tradename Novrad (a reversal of Darvon) as an antitussive.[1][2] Unlike many antitussives, it binds poorly to the sigma-1 receptor.[3]
Synthesis
[edit]
- Friedel-Crafts acylation of benzene with propionyl chloride and aluminium chloride as a Lewis acid.
- Mannich reaction of propiophenone with formaldehyde and dimethylamine affords the corresponding aminoketone.
- Reaction of the ketone with benzylmagnesium bromide gives the amino alcohol. It is of note that this intermediate fails to show analgesic activity in animal assays.
- Esterification of the alcohol by means of propionic anhydride affords the propionate.[5]
Chirality
[edit]The presence of two chiral centers in this molecule means that the compound can exist as any of four isomers. The biologic activity has been found to be associated with the α-isomer. Resolution of that isomer into its optical antipodes showed the d isomer to be the active analgesic; this is now denoted as propoxyphene. The l isomer is almost devoid of analgesic activity; the compound does, however, show useful antitussive activity and is named levopropoxyphene.[citation needed]
References
[edit]- ^ Reference.MD: Propoxyphene napsylate
- ^ Lutje Spelberg, Jeffrey Harald (2003). Enantioselective biocatalytic conversions of epoxides. Rijksuniversiteit Groningen.
- ^ Klein M, Musacchio JM (October 10, 1988). "Dextromethorphan binding sites in the guinea pig brain". Cellular and Molecular Neurobiology. 8 (2): 149–156. doi:10.1007/BF00711241. PMID 3044591. S2CID 33844132.
- ^ Chenier, Philip J. (2002) Survey of Industrial Chemistry. Springer. ISBN 0306472465. p. 455.
- ^ Pohland A, Sullivan HR (1953). "Analgesics: Esters of 4-Dialkylamino-1,2-diphenyl-2-butanols" (PDF). Journal of the American Chemical Society. 75 (18): 4458–4461. doi:10.1021/ja01114a019. Archived from the original (PDF) on 2014-11-07.
