Cancer immunotherapy
| Cancer immunotherapy | |
|---|---|
 | |
| Specialty | Immuno-oncology |
Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease.[1] It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.
Cancer immunotherapy exploits the fact that cancer cells often have tumor antigens, molecules on their surface that can bind to antibody proteins or T-cell receptors, triggering an immune system response. The tumor antigens are often proteins or other macromolecules (e.g., carbohydrates). Normal antibodies bind to external pathogens, but the modified immunotherapy antibodies bind to the tumor antigens marking and identifying the cancer cells for the immune system to inhibit or kill. The clinical success of cancer immunotherapy is highly variable between different forms of cancer; for instance, certain subtypes of gastric cancer react well to the approach whereas immunotherapy is not effective for other subtypes.[2]
In 2018, American immunologist James P. Allison and Japanese immunologist Tasuku Honjo received the Nobel Prize in Physiology or Medicine for their discovery of cancer therapy by inhibition of negative immune regulation.[3]
History
[edit]"During the 17th and 18th centuries, various forms of immunotherapy in cancer became widespread... In the 18th and 19th centuries, septic dressings enclosing ulcerative tumours were used for the treatment of cancer. Surgical wounds were left open to facilitate the development of infection, and purulent sores were created deliberately... One of the most well-known effects of microorganisms on ... cancer was reported in 1891, when an American surgeon, William Coley, inoculated patients having inoperable tumours with [ Streptococcus pyogenes ]."[4] "Coley [had] thoroughly reviewed the literature available at that time and found 38 reports of cancer patients with accidental or iatrogenic feverish erysipelas. In 12 patients, the sarcoma or carcinoma had completely disappeared; the others had substantially improved. Coley decided to attempt the therapeutic use of iatrogenic erysipelas..."[5] "Coley developed a toxin that contained heat-killed bacteria [ Streptococcus pyogenes and Serratia marcescens ]. Until 1963, this treatment was used for the treatment of sarcoma."[4] "Coley injected more than 1000 cancer patients with bacteria or bacterial products."[6] 51.9% of [Coley's] patients with inoperable soft-tissue sarcomas showed complete tumour regression and survived for more than 5 years, and 21.2% of the patients had no clinical evidence of tumour at least 20 years after this treatment..."[4] Research continued in the 20th century under Maria O'Connor Hornung at Tulane Medical School.[7][8]
Types and categories
[edit]There are several types of immunotherapy used to treat cancer:[9][10]
- Immune checkpoint inhibitors: drugs that block immune system checkpoints to allow immune cells to respond more strongly to the cancer.
- T-cell transfer therapy: a treatment that takes T-cells from the tumor and selects or changes them in the lab to better attack cancer cells, then reintroduces them into the patient.
- Monoclonal antibodies: designed to bind to specific targets on cancer cells, marking cancer cells so that they will be better seen and destroyed by the immune system.
- Treatment vaccines: also known as therapeutic cancer vaccines, help the immune system learn to recognize and react to mutant proteins specific to the tumor and destroy cancer cells containing them.
- Immune system modulators: agents that enhance the body’s immune response against cancer.
Immunotherapies can be categorized as active or passive based on their ability to engage the host immune system against cancer.[11][12] Active immunotherapy specifically targets tumor cells via the immune system. Examples include therapeutic cancer vaccines (also known as treatment vaccines,[13] which are designed to boost the body's immune system to fight cancer), CAR-T cells, and targeted antibody therapies. In contrast, passive immunotherapy does not directly target tumor cells, but enhances the ability of the immune system to attack cancer cells. Examples include checkpoint inhibitors and cytokines.
Active cellular therapies aim to destroy cancer cells by recognition of distinct markers known as antigens. In cancer vaccines, the goal is to generate an immune response to these antigens through a vaccine. Currently, only one vaccine (sipuleucel-T for prostate cancer) has been approved. In cell-mediated therapies like CAR-T cell therapy, immune cells are extracted from the patient, genetically engineered to recognize tumor-specific antigens, and returned to the patient. Cell types that can be used in this way are natural killer (NK) cells, lymphokine-activated killer cells, cytotoxic T cells, and dendritic cells. Finally, specific antibodies can be developed that recognize cancer cells and target them for destruction by the immune system. Examples of such antibodies include rituximab (targeting CD-20), trastuzumab (targeting HER-2), and cetuximab (targeting EGFR).
Passive antibody therapies aim to increase the activity of the immune system without specifically targeting cancer cells. For example, cytokines directly stimulate the immune system and increase immune activity. Checkpoint inhibitors target proteins (immune checkpoints) that normally dampen the immune response. This enhances the ability of the immune system to attack cancer cells. Current research is identifying new potential targets to enhance immune function. Approved checkpoint inhibitors include antibodies such as ipilimumab, nivolumab, and pembrolizumab.
Cellular immunotherapy
[edit]Dendritic cell therapy
[edit]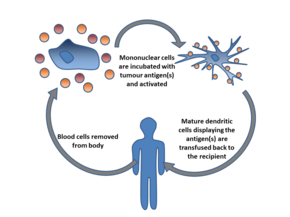
Dendritic cell therapy provokes anti-tumor responses by causing dendritic cells to present tumor antigens to lymphocytes, which activates them, priming them to kill other cells that present the antigen. Dendritic cells are antigen-presenting cells (APCs) in the mammalian immune system.[14] In cancer treatment, they aid cancer antigen targeting.[15] The only approved cellular cancer therapy based on dendritic cells is sipuleucel-T.
One method of inducing dendritic cells to present tumor antigens is by vaccination with autologous tumor lysates[16] or short peptides (small parts of the protein that correspond to the protein antigens on cancer cells). These peptides are often given in combination with adjuvants (highly immunogenic substances) to increase the immune and anti-tumor responses. Other adjuvants include proteins or other chemicals that attract and/or activate dendritic cells, such as granulocyte-macrophage colony-stimulating factor (GM-CSF). The most common sources of antigens used for dendritic cell vaccine in glioblastoma (GBM) as an aggressive brain tumor were whole tumor lysate, CMV antigen RNA and tumor-associated peptides like EGFRvIII.[17]
Dendritic cells can also be activated in vivo by making tumor cells express GM-CSF. This can be achieved by either genetically engineering tumor cells to produce GM-CSF or by infecting tumor cells with an oncolytic virus that expresses GM-CSF.
Another strategy is to remove dendritic cells from the blood of a patient and activate them outside the body. The dendritic cells are activated in the presence of tumor antigens, which may be a single tumor-specific peptide/protein or a tumor cell lysate (a solution of broken-down tumor cells). These cells (with optional adjuvants) are infused and provoke an immune response.
Dendritic cell therapies include the use of antibodies that bind to receptors on the surface of dendritic cells. Antigens can be added to the antibody and can induce the dendritic cells to mature and provide immunity to the tumor. Dendritic cell receptors such as TLR3, TLR7, TLR8 or CD40 have been used as antibody targets.[15] Dendritic cell-NK cell interface also has an important role in immunotherapy. The design of new dendritic cell-based vaccination strategies should also encompass NK cell-stimulating potency. It is critical to systematically incorporate NK cells monitoring as an outcome in antitumor DC-based clinical trials.[citation needed]
Drugs
[edit]Sipuleucel-T (Provenge) was approved for treatment of asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer in 2010. The treatment consists of removal of antigen-presenting cells from blood by leukapheresis and growing them with the fusion protein PA2024 made from GM-CSF and prostate-specific prostatic acid phosphatase (PAP) and reinfused. This process is repeated three times.[18][19][20][21]
Adoptive T-cell therapy
[edit]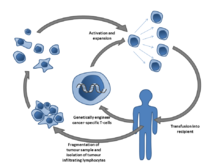
Adoptive T cell therapy is a form of passive immunization by the transfusion of T-cells. They are found in blood and tissue and typically activate when they find foreign pathogens. Activation occurs when the T-cell's surface receptors encounter cells that display parts of foreign proteins (either on their surface or intracellularly). These can be either infected cells or other antigen-presenting cells (APCs). The latter are found in normal tissue and in tumor tissue, where they are known as tumor-infiltrating lymphocytes (TILs). They are activated by the presence of APCs such as dendritic cells that present tumor antigens. Although these cells can attack tumors, the tumor microenvironment is highly immunosuppressive, interfering with immune-mediated tumour death.[22]
Multiple ways of producing tumour-destroying T-cells have been developed. Most commonly, T-cells specific to a tumor antigen can be removed from a tumor sample (TILs) or filtered from blood. The T-cells can optionally be modified in various ways, cultured and infused into patients. T cells can be modified via genetic engineering, producing CAR-T cell or TCR T cells or by exposing the T cells to tumor antigens in a non-immunosuppressive environment, that they recognize as foreign and learn to attack.
Another approach is transfer of haploidentical γδ T cells or natural killer cells from a healthy donor.[23] The major advantage of this approach is that these cells do not cause graft-versus-host disease. The disadvantage is that transferred cells frequently have impaired function.[24]
Tumor-derived T cell therapy
[edit]The simplest example involves removing TILs from a tumor, culturing but not modifying them, and infusing the result back into the tumour. The first therapy of this type, Lifileucel, achieved US Food and Drug Administration (FDA) approval in February 2024.
CAR-T cell therapy
[edit]The premise of CAR-T immunotherapy is to modify T cells to recognize cancer cells in order to target and destroy them. Scientists harvest T cells from people, genetically alter them to add a chimeric antigen receptor (CAR) that specifically recognizes cancer cells, then infuse the resulting CAR-T cells into patients to attack their tumors.
Tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR-T) therapy, was approved by the FDA in 2017 to treat acute lymphoblastic leukemia (ALL).[25] This treatment removes CD19 positive cells (B-cells) from the body (including the diseased cells, but also normal antibody-producing cells).
Axicabtagene ciloleucel (Yescarta) is another CAR-T therapeutic, approved in 2017 for treatment of diffuse large B-cell lymphoma (DLBCL).[26]
Multifunctional alginate scaffolds
[edit]Multifunctional alginate scaffolds for T cell engineering and release (MASTER) is a technique for in situ engineering, replication and release of genetically engineered T cells. It is an evolution of CAR T cell therapy. T cells are extracted from the patient and mixed with a genetically engineered virus that contains a cancer-targeting gene (as with CAR T). The mixture is then added to a MASTER (scaffold), which absorbs them. The MASTER contains antibodies that activate the T cells and interleukins that trigger cell proliferation. The MASTER is then implanted into the patient. The activated T cells interact with the viruses to become CAR T cells. The interleukins stimulate these CAR T cells to proliferate, and the CAR T cells exit the MASTER to attack the cancer. The technique takes hours instead of weeks. And because the cells are younger, they last longer in the body, show stronger potency against cancer, and display fewer markers of exhaustion. These features were demonstrated in mouse models. The treatment was more effective and longer-lasting against lymphoma.[27][28]
T cell receptor T cell therapy
[edit]T cell receptor T cell therapy (TCR-T) is a type of adoptive T-cell therapy that targets some cancers. TCR-T therapies use heterodimers made of alpha and beta peptide chains to recognize MHC-presented polypeptide fragment molecules. Unlike CAR-T, which uses cell surface antigens, TCR-T can recognize MHC's larger set of intracellular antigen fragments. However, TCR-T cell therapy depends on MHC molecules, limiting its usefulness.[29][30]
Each T cell's TCR is specific to one antigen and sits on the T cell's surface. The affinity of human TCRs to tumor antigens is relatively low, rendering them unable to recognize and kill tumor cells effectively. The modified T cell has much higher affinity, which enhances both recognition and affinity supporting the recognition of tumor cells.[29]Antibody therapy
[edit]
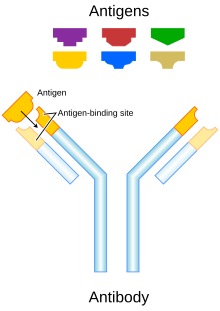
Antibody types
[edit]Conjugation
[edit]Two types are used in cancer treatments:[32]
- Naked monoclonal antibodies are antibodies without added elements. Most antibody therapies use this antibody type.
- Conjugated monoclonal antibodies are joined to another molecule, which is either cytotoxic or radioactive. The toxic chemicals are those typically used as chemotherapy drugs, but other toxins can be used. The antibody binds to specific antigens on cancer cell surfaces, directing the therapy to the tumor. Radioactive compound-linked antibodies are referred to as radiolabelled. Chemolabelled or immunotoxins antibodies are tagged with chemotherapeutic molecules or toxins, respectively.[33] Research has also demonstrated conjugation of a TLR agonist to an anti-tumor monoclonal antibody.[34]
Fc regions
[edit]Fc's ability to bind Fc receptors is important because it allows antibodies to activate the immune system. Fc regions are varied: they exist in numerous subtypes and can be further modified, for example with the addition of sugars in a process called glycosylation. Changes in the Fc region can alter an antibody's ability to engage Fc receptors and, by extension, will determine the type of immune response that the antibody triggers.[35] For example, immune checkpoint blockers targeting PD-1 are antibodies designed to bind PD-1 expressed by T cells and reactivate these cells to eliminate tumors.[36] Anti-PD-1 drugs contain not only a Fab region that binds PD-1 but also an Fc region. Experimental work indicates that the Fc portion of cancer immunotherapy drugs can affect the outcome of treatment. For example, anti-PD-1 drugs with Fc regions that bind inhibitory Fc receptors can have decreased therapeutic efficacy.[37] Imaging studies have further shown that the Fc region of anti-PD-1 drugs can bind Fc receptors expressed by tumor-associated macrophages. This process removes the drugs from their intended targets (i.e. PD-1 molecules expressed on the surface of T cells) and limits therapeutic efficacy.[38] Furthermore, antibodies targeting the co-stimulatory protein CD40 require engagement with selective Fc receptors for optimal therapeutic efficacy.[39] Together, these studies underscore the importance of Fc status in antibody-based immune checkpoint targeting strategies.
Human/non-human antibodies
[edit]Antibodies can come from a variety of sources, including human cells, mice, and a combination of the two (chimeric antibodies). Different sources of antibodies can provoke different kinds of immune responses. For example, the human immune system can recognize mouse antibodies (also known as murine antibodies) and trigger an immune response against them. This could reduce the effectiveness of the antibodies as a treatment and cause an immune reaction. Chimeric antibodies attempt to reduce murine antibodies' immunogenicity by replacing part of the antibody with the corresponding human counterpart. Humanized antibodies are almost completely human; only the complementarity determining regions of the variable regions are derived from murine sources. Human antibodies have been produced using unmodified human DNA.[33]

Mechanism of action
[edit]Antibody-dependent cell-mediated cytotoxicity (ADCC)
[edit]Antibody-dependent cell-mediated cytotoxicity (ADCC) requires antibodies to bind to target cell surfaces. Antibodies are formed of a binding region (Fab) and the Fc region that can be detected by immune system cells via their Fc surface receptors. Fc receptors are found on many immune system cells, including NK cells. When NK cells encounter antibody-coated cells, the latter's Fc regions interact with their Fc receptors, releasing perforin and granzyme B to kill the tumor cell. Examples include rituximab, ofatumumab, elotuzumab, and alemtuzumab. Antibodies under development have altered Fc regions that have higher affinity for a specific type of Fc receptor, FcγRIIIA, which can dramatically increase effectiveness.[40][41]
Anti-CD47 therapy
[edit]Many tumor cells overexpress CD47 to escape immunosurveilance of host immune system. CD47 binds to its receptor signal-regulatory protein alpha (SIRPα) and downregulate phagocytosis of tumor cell.[42] Therefore, anti-CD47 therapy aims to restore clearance of tumor cells. Additionally, growing evidence supports the employment of tumor antigen-specific T cell response in response to anti-CD47 therapy.[43][44] A number of therapeutics are being developed, including anti-CD47 antibodies, engineered decoy receptors, anti-SIRPα antibodies and bispecific agents.[43] As of 2017, wide range of solid and hematologic malignancies were being clinically tested.[43][45]
Anti-GD2 antibodies
[edit]
Carbohydrate antigens on the surface of cells can be used as targets for immunotherapy. GD2 is a ganglioside found on the surface of many types of cancer cell including neuroblastoma, retinoblastoma, melanoma, small cell lung cancer, brain tumors, osteosarcoma, rhabdomyosarcoma, Ewing's sarcoma, liposarcoma, fibrosarcoma, leiomyosarcoma and other soft tissue sarcomas. It is not usually expressed on the surface of normal tissues, making it a good target for immunotherapy. As of 2014, clinical trials were underway.[46]
Complement Activation
[edit]The complement system includes blood proteins that can cause cell death after an antibody binds to the cell surface (the classical complement pathway, among the ways of complement activation). Generally, the system deals with foreign pathogens but can be activated with therapeutic antibodies in cancer. The system can be triggered if the antibody is chimeric, humanized, or human; as long as it contains the IgG1 Fc region. Complement can lead to cell death by activation of the membrane attack complex, known as complement-dependent cytotoxicity; enhancement of antibody-dependent cell-mediated cytotoxicity; and CR3-dependent cellular cytotoxicity. Complement-dependent cytotoxicity occurs when antibodies bind to the cancer cell surface, the C1 complex binds to these antibodies and subsequently, protein pores are formed in cancer cell membrane.[47]
Blocking
Antibody therapies can also function by binding to proteins and physically blocking them from interacting with other proteins. Checkpoint inhibitors (CTLA-4, PD-1, and PD-L1) operate by this mechanism. Briefly, checkpoint inhibitors are proteins that normally help to slow immune responses and prevent the immune system from attacking normal cells. Checkpoint inhibitors bind these proteins and prevent them from functioning normally, which increases the activity of the immune system. Examples include durvalumab, ipilimumab, nivolumab, and pembrolizumab.
FDA-approved antibodies
[edit]| Antibody | Brand name | Type | Target | Approval date | Approved treatment(s) |
|---|---|---|---|---|---|
| Alemtuzumab | Campath | humanized | CD52 | 2001 | B-cell chronic lymphocytic leukemia (CLL)[49] |
| Atezolizumab | Tecentriq | humanized | PD-L1 | 2016 | bladder cancer[50] |
| Atezolizumab/hyaluronidase | Tecentriq Hybreza | humanized | PD-L1 | 2024 | non-small cell lung cancer, small cell lung cancer, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma[51][52][53] |
| Avelumab | Bavencio | human | PD-L1 | 2017 | metastatic Merkel cell carcinoma[54] |
| Durvalumab | Imfinzi | human | PD-L1 | 2017 | bladder cancer[55] non-small cell lung cancer[56] |
| Elotuzumab | Empliciti | humanized | SLAMF7 | 2015 | multiple myeloma[57] |
| Ipilimumab | Yervoy | human | CTLA4 | 2011 | metastatic melanoma[58] |
| Nivolumab | Opdivo | human | PD-1 | 2014 | unresectable or metastatic melanoma, squamous non-small cell lung cancer, Renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, classical hodgkin lymphoma[59][60] |
| Ofatumumab | Arzerra | human | CD20 | 2009 | refractory CLL[61] |
| Pembrolizumab | Keytruda | humanized | PD-1 | 2014 | unresectable or metastatic melanoma, squamous non-small cell lung cancer (NSCLC),[62] Hodgkin's lymphoma,[63] Merkel-cell carcinoma (MCC),[64] primary mediastinal B-cell lymphoma (PMBCL),[65] stomach cancer, cervical cancer[66] |
| Rituximab | Rituxan, Mabthera | chimeric | CD20 | 1997 | non-Hodgkin lymphoma[67] |
| Rituximab/hyaluronidase | Rituxan Hycela | chimeric | CD20 | 2017 | follicular lymphoma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia[68] |
| Trastuzumab | Rituxan Hycela | humanized | HER2/neu | 1998 | breast cancer, gastric or gastroesophageal junction adenocarcinoma |
Alemtuzumab
[edit]Alemtuzumab (Campath-1H) is an anti-CD52 humanized IgG1 monoclonal antibody indicated for the treatment of fludarabine-refractory chronic lymphocytic leukemia (CLL), cutaneous T-cell lymphoma, peripheral T-cell lymphoma and T-cell prolymphocytic leukemia. CD52 is found on >95% of peripheral blood lymphocytes (both T-cells and B-cells) and monocytes, but its function in lymphocytes is unknown. It binds to CD52 and initiates its cytotoxic effect by complement fixation and ADCC mechanisms. Due to the antibody target (cells of the immune system), common complications of alemtuzumab therapy are infection, toxicity and myelosuppression.[69][70][71]
Atezolizumab
[edit]
Atezolizumab, sold under the brand name Tecentriq among others, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma and alveolar soft part sarcoma,[72][73] but discontinued for use in triple-negative breast cancer (TNBC).[74] It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).[75]
The most common side effects when used on its own include tiredness, reduced appetite, nausea, vomiting, cough, difficulty breathing, diarrhea, rash, fever, pain in the back, joints, muscles and bones, weakness, itching and urinary tract infection.[76] The most common side effects when used with other cancer medicines include peripheral neuropathy (nerve damage in the hands and feet), nausea, anemia (low red blood cell counts), neutropenia (low white blood cell counts), thrombocytopenia (low platelet counts), rash, tiredness, constipation, reduced appetite, diarrhea, and cough.[76]
Atezolizumab was the first PD-L1 inhibitor approved by the U.S. for bladder cancer. Food and Drug Administration (FDA).[77]
In the European Union, atezolizumab is the first PD-(L)1 cancer immunotherapy for subcutaneous injection.[78]Atezolizumab/hyaluronidase
[edit]
Atezolizumab/hyaluronidase, sold under the brand name Tecentriq Hybreza, is a fixed-dose combination medication used for the treatment of non-small cell lung cancer, small cell lung cancer, hepatocellular carcinoma, melanoma, and alveolar soft part sarcoma.[79][80] It contains atezolizumab, a programmed death-ligand 1 (PD-L1) blocking monoclonal antibody; and hyaluronidase (human recombinant), an endoglycosidase.[79] It is taken by subcutaneous injection.[79]
The most common adverse reactions include fatigue, musculoskeletal pain, cough, dyspnea, and decreased appetite.[79][80]
Atezolizumab/hyaluronidase was approved for medical use in the United States in September 2024.[79][80][81][82]Avelumab
[edit]
Avelumab, sold under the brand name Bavencio, is a fully human monoclonal antibody medication for the treatment of Merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma.[83]
Common side effects include fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reactions, rash, decreased appetite and swelling of the limbs (peripheral edema).[84]
Avelumab targets the protein programmed death-ligand 1 (PD-L1). It has received orphan drug designation by the European Medicines Agency (EMA) for the treatment of gastric cancer in January 2017.[85] The US Food and Drug Administration (FDA) approved it in March 2017, for the treatment of Merkel-cell carcinoma,[84] an aggressive type of skin cancer. The EMA approved it in September 2017, for the same indication.[86] This is the first FDA-approved treatment for metastatic Merkel-cell carcinoma, a rare, aggressive form of skin cancer.[84] Avelumab was developed by Merck KGaA and Pfizer.[87]Durvalumab
[edit]Durvalumab (Imfinzi) is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that blocks the interaction of programmed cell death ligand 1 (PD-L1) with the PD-1 and CD80 (B7.1) molecules. Durvalumab is approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma who:
- have disease progression during or following platinum-containing chemotherapy.
- have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
On 16 February 2018, the Food and Drug Administration approved durvalumab for patients with unresectable stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.[88]
Elotuzumab
[edit]| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | SLAMF7 (CD319) |
| Clinical data | |
| Trade names | Empliciti |
| Other names | HuLuc63 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Identifiers | |
| CAS Number | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6476H9982N1714O2016S42 |
| Molar mass | 145453.59 g·mol−1 |
| | |
Elotuzumab, sold under the brand name Empliciti, is a humanized IgG1 monoclonal antibody medication used in combination with lenalidomide and dexamethasone, for adults that have received 1 to 3 prior therapies for the treatment of multiple myeloma.[91] It is also indicated for adult patients in combination with pomalidomide and dexamethasone, who have received 2 prior therapies including lenalidomide and a protease inhibitor.[91] Administration of elotuzumab is done intravenously.[91] Each intravenous injection of elotuzumab should be premedicated with dexamethasone, diphenhydramine, ranitidine and acetaminophen.[92] It is being developed by Bristol Myers Squibb and AbbVie.[93]
Common side effects of elotuzumab with lenalidomide and dexamethasone includes fatigue, diarrhea, pyrexia, constipation, cough, peripheral neuropathy, nasopharyngitis, upper respiratory tract infection, decreased appetite, and pneumonia.[91] The most common side effects of elotuzumab with pomalidomide and dexamethasone includes constipation and hyperglycemia.[91] There is no available information for the use of elotuzumab in pregnant women.[91]
Elotuzumab is an immunostimulatory antibody that targets the Signaling Lymphocytic Activation Molecule Family member 7 (SLAMF7) through two mechanisms.[91]
In May 2014, it was granted breakthrough therapy designation by the US Food and Drug Administration (FDA) (for multiple myeloma).[94] The initial FDA approval of elotuzumab in 2015 in combination with lenalidomide and dexamethasone was carried out through the results illustrated in the ELOQUENT 2 study.[95] In May 2016 the EC/EU gave a similar approval.[96] Furthermore, the results of the ELOQUENT 3 study led to the FDA approval of elotuzumab in combination with pomalidomide and dexamethasone in 2018.[97]Ipilimumab
[edit]Ipilimumab (Yervoy) is a human IgG1 antibody that binds the surface protein CTLA4. In normal physiology T-cells are activated by two signals: the T-cell receptor binding to an antigen-MHC complex and T-cell surface receptor CD28 binding to CD80 or CD86 proteins. CTLA4 binds to CD80 or CD86, preventing the binding of CD28 to these surface proteins and therefore negatively regulates the activation of T-cells.[98][99][100][101]
Active cytotoxic T-cells are required for the immune system to attack melanoma cells. Normally inhibited active melanoma-specific cytotoxic T-cells can produce an effective anti-tumor response. Ipilimumab can cause a shift in the ratio of regulatory T-cells to cytotoxic T-cells to increase the anti-tumor response. Regulatory T-cells inhibit other T-cells, which may benefit the tumor.[98][99][100][101]
Nivolumab
[edit]Nivolumab is a human IgG4 antibody that prevents T-cell inactivation by blocking the binding of programmed cell death 1 ligand 1 or programmed cell death 1 ligand 2 (PD-L1 or PD-L2), a protein expressed by cancer cells, with PD-1, a protein found on the surface of activated T-cells.[102][103] Nivolumab is used in advanced melanoma, metastatic renal cell carcinoma, advanced lung cancer, advanced head and neck cancer, and Hodgkin's lymphoma.[104]
Ofatumumab
[edit]Ofatumumab is a second generation human IgG1 antibody that binds to CD20. It is used in the treatment of chronic lymphocytic leukemia (CLL) because the cancerous cells of CLL are usually CD20-expressing B-cells. Unlike rituximab, which binds to a large loop of the CD20 protein, ofatumumab binds to a separate, small loop. This may explain their different characteristics. Compared to rituximab, ofatumumab induces complement-dependent cytotoxicity at a lower dose with less immunogenicity.[105][106]
Pembrolizumab
[edit]As of 2019, pembrolizumab, which blocks PD-1, programmed cell death protein 1, has been used via intravenous infusion to treat inoperable or metastatic melanoma, metastatic non-small cell lung cancer (NSCLC) in certain situations, as a second-line treatment for head and neck squamous cell carcinoma (HNSCC), after platinum-based chemotherapy, and for the treatment of adult and pediatric patients with refractory classic Hodgkin's lymphoma (cHL).[107][108] It is also indicated for certain patients with urothelial carcinoma, stomach cancer and cervical cancer.[109]
Rituximab
[edit]Rituximab is a chimeric monoclonal IgG1 antibody specific for CD20, developed from its parent antibody Ibritumomab. As with ibritumomab, rituximab targets CD20, making it effective in treating certain B-cell malignancies. These include aggressive and indolent lymphomas such as diffuse large B-cell lymphoma and follicular lymphoma and leukemias such as B-cell chronic lymphocytic leukemia. Although the function of CD20 is relatively unknown, CD20 may be a calcium channel involved in B-cell activation. The antibody's mode of action is primarily through the induction of ADCC and complement-mediated cytotoxicity. Other mechanisms include apoptosis[clarification needed] and cellular growth arrest. Rituximab also increases the sensitivity of cancerous B-cells to chemotherapy.[110][111][112][113][114]
Trastuzumab
[edit]
Trastuzumab, sold under the brand name Herceptin among others, is a monoclonal antibody used to treat breast cancer and stomach cancer.[143][140][144][145] It is specifically used for cancer that is HER2 receptor positive.[143] It may be used by itself or together with other chemotherapy medication.[143] Trastuzumab is given by slow injection into a vein and injection just under the skin.[143][146]
Common side effects include fever, infection, cough, headache, trouble sleeping, and rash.[143] Other severe side effects include heart failure, allergic reactions, and lung disease.[143] Use during pregnancy may harm the baby.[136] Trastuzumab works by binding to the HER2 receptor and slowing down cell replication.[143]
Trastuzumab was approved for medical use in the United States in September 1998, and in the European Union in August 2000.[147][145] It is on the World Health Organization's List of Essential Medicines.[148]Immune checkpoint antibody therapy or immune checkpoint blockade
[edit]

Immune checkpoints affect the immune system function. Immune checkpoints can be stimulatory or inhibitory. Tumors can use these checkpoints to protect themselves from immune system attacks. Checkpoint therapies approved as of 2012 block inhibitory checkpoint receptors. Blockade of negative feedback signaling to immune cells thus results in an enhanced immune response against tumors.[103] As of 2020, immune checkpoint blockade therapies have varied effectiveness. In Hodgkin lymphoma and natural killer T-cell lymphoma, response rates are high, at 50–60%. Response rates are quite low for breast and prostate cancers, however.[149] A major challenge are the large variations in responses to immunocheckpoint inhibitors, some patients showing spectacular clinical responses while no positive effects are seen in others. A plethora of possible reasons for the absence of efficacy in many patients have been proposed, but the biomedical community has still to begin to find consensus in this respect. For instance, a recent paper documented that infection with Helicobacter pylori would negatively influence the effects of immunocheckpoint inhibitors in gastric cancer.,[150] but this notion was quickly challenged by others.[151]
One ligand-receptor interaction under investigation is the interaction between the transmembrane programmed cell death 1 protein (PDCD1, PD-1; also known as CD279) and its ligand, PD-1 ligand 1 (PD-L1, CD274). PD-L1 on the cell surface binds to PD1 on an immune cell surface, which inhibits immune cell activity. Among PD-L1 functions is a key regulatory role on T cell activities. It appears that (cancer-mediated) upregulation of PD-L1 on the cell surface may inhibit T cells that might otherwise attack. PD-L1 on cancer cells also inhibits FAS- and interferon-dependent apoptosis, protecting cells from cytotoxic molecules produced by T cells. Antibodies that bind to either PD-1 or PD-L1 and therefore block the interaction may allow the T-cells to attack the tumor.[152]
CTLA-4 blockade
[edit]The first checkpoint antibody approved by the FDA was ipilimumab, approved in 2011 to treat melanoma.[153] It blocks the immune checkpoint molecule CTLA-4. As of 2012, clinical trials have also shown some benefits of anti-CTLA-4 therapy on lung cancer or pancreatic cancer, specifically in combination with other drugs.[154][155] In on-going trials the combination of CTLA-4 blockade with PD-1 or PD-L1 inhibitors is tested on different types of cancer.[156]
However, as of 2015 it is known that patients treated with checkpoint blockade (specifically CTLA-4 blocking antibodies), or a combination of check-point blocking antibodies, are at high risk of having immune-related adverse events such as dermatologic, gastrointestinal, endocrine, or hepatic autoimmune reactions.[102] These are most likely due to the breadth of the induced T-cell activation when anti-CTLA-4 antibodies are administered by injection in the bloodstream.
A 2024 cohort study of ICI use during pregnancy showed no overreporting of specific adverse effects on pregnancy, fetal, and/or newborn outcomes, interestingly.[157]
Using a mouse model of bladder cancer, researchers have found that a local injection of a low dose anti-CTLA-4 in the tumour area had the same tumour inhibiting capacity as when the antibody was delivered in the blood.[158] At the same time the levels of circulating antibodies were lower, suggesting that local administration of the anti-CTLA-4 therapy might result in fewer adverse events.[158]
PD-1 inhibitors
[edit]Initial clinical trial results with IgG4 PD1 antibody nivolumab were published in 2010.[103] It was approved in 2014. Nivolumab is approved to treat melanoma, lung cancer, kidney cancer, bladder cancer, head and neck cancer, and Hodgkin's lymphoma.[159] A 2016 clinical trial for non-small cell lung cancer failed to meet its primary endpoint for treatment in the first-line setting, but is FDA-approved in subsequent lines of therapy.[160]
Pembrolizumab (Keytruda) is another PD1 inhibitor that was approved by the FDA in 2014. Pembrolizumab is approved to treat melanoma and lung cancer.[159]
Antibody BGB-A317 is a PD-1 inhibitor (designed to not bind Fc gamma receptor I) in early clinical trials.[161]
PD-L1 inhibitors
[edit]In May 2016, PD-L1 inhibitor atezolizumab[162] was approved for treating bladder cancer.
Anti-PD-L1 antibodies currently in development include avelumab[163] and durvalumab,[164] in addition to an inhibitory affimer.[165]
CISH
[edit]Combinations
[edit]Many cancer patients do not respond to immune checkpoint blockade. Response rate may be improved by combining that with additional therapies, including those that stimulate T cell infiltration. For example, targeted therapies such as radiotherapy, vasculature targeting agents, and immunogenic chemotherapy[170] can improve immune checkpoint blockade response in animal models.
Combining immunotherapies such as PD1 and CTLA4 inhibitors can create to durable responses.[171][172]
Combinatorial ablation and immunotherapy enhances the immunostimulating response and has synergistic effects for metastatic cancer treatment.[173]
Combining checkpoint immunotherapies with pharmaceutical agents has the potential to improve response, and as of 2018 were a target of clinical investigation.[174] Immunostimulatory drugs such as CSF-1R inhibitors and TLR agonists have been effective.[175][176]
Two independent 2024 clinical trials reported that combinations of JAK inhibitors with anti–PD-1 immunotherapy could improve efficacy. A phase 2 trial investigated the combination as a first-line therapy for metastatic non-small-cell lung cancer. Administration of itacitinib after treatment with pembrolizumab improved therapeutic response. A separate phase 1/2 trial with patients with relapsed/refractory Hodgkin’s lymphoma combined ruxolitinib and nivolumab, yielding improved clinical efficacy in patients who had previously failed checkpoint blockade immunotherapy.[177]
Cytokine therapy
[edit]Cytokines are proteins produced by many types of cells present within a tumor. They can modulate immune responses. The tumor often employs them to allow it to grow and reduce the immune response. These immune-modulating effects allow them to be used as drugs to provoke an immune response. Two commonly used cytokines are interferons and interleukins.[178]
Interleukin-2 and interferon-α are cytokines, proteins that regulate and coordinate the behavior of the immune system. They have the ability to enhance anti-tumor activity and thus can be used as passive cancer treatments. Interferon-α is used in the treatment of hairy-cell leukaemia, AIDS-related Kaposi's sarcoma, follicular lymphoma, chronic myeloid leukaemia and malignant melanoma. Interleukin-2 is used in the treatment of malignant melanoma and renal cell carcinoma.[179]
Interferon
[edit]Interferons are produced by the immune system. They are usually involved in anti-viral response, but also have use for cancer. They fall in three groups: type I (IFNα and IFNβ), type II (IFNγ) and type III (IFNλ). IFNα has been approved for use in hairy-cell leukaemia, AIDS-related Kaposi's sarcoma, follicular lymphoma, chronic myeloid leukaemia and melanoma. Type I and II IFNs have been researched extensively and although both types promote anti-tumor immune system effects, only type I IFNs have been shown to be clinically effective. IFNλ shows promise for its anti-tumor effects in animal models.[180][181]
Unlike type I IFNs, Interferon gamma is not approved yet for the treatment of any cancer. However, improved survival was observed when Interferon gamma was administered to patients with bladder carcinoma and melanoma cancers. The most promising result was achieved in patients with stage 2 and 3 of ovarian carcinoma. The in vitro study of IFN-gamma in cancer cells is more extensive and results indicate anti-proliferative activity of IFN-gamma leading to the growth inhibition or cell death, generally induced by apoptosis but sometimes by autophagy.[182]
Interleukin
[edit]Interleukins have an array of immune system effects. Interleukin-2 is used in the treatment of malignant melanoma and renal cell carcinoma. In normal physiology it promotes both effector T cells and T-regulatory cells, but its exact mechanism of action is unknown.[178][183]
Genetic pre-treatment testing for therapeutic significance
[edit]Because of the high cost of many of immunotherapy medications and the reluctance of medical insurance companies to prepay for their prescriptions various test methods have been proposed, to attempt to forecast the effectiveness of these medications. In some cases the FDA has approved genetic tests for medication specific to certain genetic markers. For example, the FDA approved BRAF-associated medication for metastatic melanoma, to be administered to patients after testing for the BRAF genetic mutation.[184]
As of 2018, the detection of PD-L1 protein seemed to be an indication of cancer susceptible to several immunotherapy medications, but research found that both the lack of this protein or its inclusion in the cancerous tissue was inconclusive, due to the little-understood varying quantities of the protein during different times and locations within the infected cells and tissue.[185][186][187]
In 2018, some genetic indications such as Tumor Mutational Burden (TMB, the number of mutations within a targeted genetic region in the cancerous cell's DNA), and microsatellite instability (MSI, the quantity of impaired DNA mismatch leading to probable mutations), have been approved by the FDA as good indicators for the probability of effective treatment of immunotherapy medication for certain cancers, but research is still in progress.[188][189] As of 2020, the patient prioritization for immunotherapy based on TMB was still highly controversial.[190][191]
Tests of this sort are being widely advertised for general cancer treatment and are expensive. In the past, some genetic testing for cancer treatment has been involved in scams such as the Duke University Cancer Fraud scandal, or claimed to be hoaxes.[192][193][194]
Research
[edit]Oncolytic virus
[edit]An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis, they release new infectious virus particles or virions to help destroy the remaining tumour. Oncolytic viruses are thought not only to cause direct destruction of the tumour cells, but also to stimulate host anti-tumour immune responses for long-term immunotherapy.[195][196][197]
The potential of viruses as anti-cancer agents was first realized in the early twentieth century, although coordinated research efforts did not begin until the 1960s. A number of viruses including adenovirus, reovirus, measles, herpes simplex, Newcastle disease virus and vaccinia have now been clinically tested as oncolytic agents. T-Vec is the first FDA-approved oncolytic virus for the treatment of melanoma. A number of other oncolytic viruses are in Phase II-III development.[198]
Polysaccharides
[edit]Certain compounds found in mushrooms, primarily polysaccharides, can up-regulate the immune system and may have anti-cancer properties. For example, beta-glucans such as lentinan have been shown in laboratory studies to stimulate macrophage, NK cells, T cells and immune system cytokines and have been investigated in clinical trials as immunologic adjuvants.[199]
Neoantigens
[edit]Many tumors express mutations. These mutations potentially create new targetable antigens (neoantigens) for use in T-cell immunotherapy. The presence of CD8+ T cells in cancer lesions, as identified using RNA sequencing data, is higher in tumors with a high mutational burden. The level of transcripts associated with the cytolytic activity of natural killer cells and T cells positively correlates with mutational load in many human tumors. In non–small cell lung cancer patients treated with lambrolizumab, mutational load shows a strong correlation with clinical response. In melanoma patients treated with ipilimumab, the long-term benefit is also associated with a higher mutational load, although less significantly. The predicted MHC binding neoantigens in patients with a long-term clinical benefit were enriched for a series of tetrapeptide motifs that were not found in tumors of patients with no or minimal clinical benefit.[200] However, human neoantigens identified in other studies do not show the bias toward tetrapeptide signatures.[201]
Polysaccharide-K
[edit]In the 1980s, Japan's Ministry of Health, Labour and Welfare approved polysaccharide-K extracted from the mushroom, Coriolus versicolor, to stimulate the immune systems of patients undergoing chemotherapy. It is a dietary supplement in the US and other jurisdictions.[202]
See also
[edit]- Cancer vaccine
- Antigen 5T4
- Coley's toxins
- Combinatorial ablation and immunotherapy
- Cryoimmunotherapy
- Photoimmunotherapy
- Radioimmunotherapy
References
[edit]- ^ Biancalana M (14 December 2022). "Harnessing the immune system to develop breakthrough cancer therapies". Archived from the original on 4 December 2023. Retrieved 19 April 2024.
- ^ Kodach LL, Peppelenbosch MP (August 2021). "Targeting the Myeloid-Derived Suppressor Cell Compartment for Inducing Responsiveness to Immune Checkpoint Blockade Is Best Limited to Specific Subtypes of Gastric Cancers". Gastroenterology. 161 (2): 727. doi:10.1053/j.gastro.2021.03.047. PMID 33798523.
- ^ "The Nobel Prize in Physiology or Medicine 2018". NobelPrize.org. Retrieved 4 August 2019.
- ^ a b c Kucerova P, Cervinkova M (April 2016). "Spontaneous regression of tumour and the role of microbial infection--possibilities for cancer treatment". Anti-Cancer Drugs. 27 (4): 269–77. doi:10.1097/CAD.0000000000000337. PMC 4777220. PMID 26813865.
- ^ Kienle GS (March 2012). "Fever in Cancer Treatment: Coley's Therapy and Epidemiologic Observations". Global Advances in Health and Medicine. 1 (1): 92–100. doi:10.7453/gahmj.2012.1.1.016. PMC 3833486. PMID 24278806.
- ^ McCarthy EF (2006). "The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas". The Iowa Orthopaedic Journal. 26: 154–8. PMC 1888599. PMID 16789469.
- ^ Dissertation Abstracts International: Retrospective Index, Volumes I-XXIX. University Microfilms. 1970.
- ^ "Commencement speakers praise, advise local graduates . . ". Washington Post. ISSN 0190-8286. Retrieved 9 July 2021.
- ^ "Immunotherapy to Treat Cancer". National Cancer Institute. 24 September 2019. Retrieved 14 October 2023.
- ^ "Immunotherapy for Cancer: An Overview". Oncodaily.com. 29 May 2024. Retrieved 29 May 2024.
- ^ Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buqué A, Senovilla L, Baracco EE, et al. (December 2014). "Classification of current anticancer immunotherapies". Oncotarget. 5 (24): 12472–12508. doi:10.18632/oncotarget.2998. PMC 4350348. PMID 25537519.
- ^ "Types of Biological Therapy". SEER Training Modules. National Cancer Institute. Retrieved 14 October 2023.
- ^ "What are Cancer Vaccines?". Cancer.Net. 30 September 2013. Retrieved 15 August 2021.
- ^ Riddell SR (July 2001). "Progress in cancer vaccines by enhanced self-presentation". Proceedings of the National Academy of Sciences of the United States of America. 98 (16): 8933–35. Bibcode:2001PNAS...98.8933R. doi:10.1073/pnas.171326398. PMC 55350. PMID 11481463.
- ^ a b Palucka K, Banchereau J (July 2013). "Dendritic-cell-based therapeutic cancer vaccines". Immunity. 39 (1): 38–48. doi:10.1016/j.immuni.2013.07.004. PMC 3788678. PMID 23890062.
- ^ Hirayama M, Nishimura Y (July 2016). "The present status and future prospects of peptide-based cancer vaccines". International Immunology. 28 (7): 319–28. doi:10.1093/intimm/dxw027. PMID 27235694.
- ^ Dastmalchi F, Karachi A, Mitchell D (June 2018). "Dendritic Cell Therapy". eLS. American Cancer Society. pp. 1–27. doi:10.1002/9780470015902.a0024243. ISBN 9780470015902. S2CID 155185753.
- ^ Gardner TA, Elzey BD, Hahn NM (April 2012). "Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer". Human Vaccines & Immunotherapeutics. 8 (4): 534–39. doi:10.4161/hv.19795. PMID 22832254.
- ^ Oudard S (May 2013). "Progress in emerging therapies for advanced prostate cancer". Cancer Treatment Reviews. 39 (3): 275–89. doi:10.1016/j.ctrv.2012.09.005. PMID 23107383.
- ^ Sims RB (June 2012). "Development of sipuleucel-T: autologous cellular immunotherapy for the treatment of metastatic castrate-resistant prostate cancer". Vaccine. 30 (29): 4394–97. doi:10.1016/j.vaccine.2011.11.058. PMID 22122856.
- ^ Shore ND, Mantz CA, Dosoretz DE, Fernandez E, Myslicki FA, McCoy C, et al. (January 2013). "Building on sipuleucel-T for immunologic treatment of castration-resistant prostate cancer". Cancer Control. 20 (1): 7–16. doi:10.1177/107327481302000103. PMID 23302902.
- ^ Restifo NP, Dudley ME, Rosenberg SA (March 2012). "Adoptive immunotherapy for cancer: harnessing the T cell response". Nature Reviews. Immunology. 12 (4): 269–81. doi:10.1038/nri3191. PMC 6292222. PMID 22437939.
- ^ Barros MS, de Araújo ND, Magalhães-Gama F, Pereira Ribeiro TL, Alves Hanna FS, Tarragô AM, et al. (22 September 2021). "γδ T Cells for Leukemia Immunotherapy: New and Expanding Trends". Frontiers in Immunology. 12: 729085. doi:10.3389/fimmu.2021.729085. PMC 8493128. PMID 34630403.
- ^ Wilhelm M, Smetak M, Schaefer-Eckart K, Kimmel B, Birkmann J, Einsele H, et al. (February 2014). "Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells". Journal of Translational Medicine. 12: 45. doi:10.1186/1479-5876-12-45. PMC 3926263. PMID 24528541.
- ^ Office of the Commissioner. "Press Announcements – FDA approval brings first gene therapy to the United States". fda.gov. Retrieved 13 December 2017.
- ^ "FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma". fda.gov. 18 October 2017. Retrieved 8 November 2017.
- ^ Irving M (29 March 2022). "Implantable immunotherapy "factory" fights cancer faster, more effectively". New Atlas. Retrieved 29 March 2022.
- ^ Agarwalla P, Ogunnaike EA, Ahn S, Froehlich KA, Jansson A, Ligler FS, et al. (March 2022). "Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells". Nature Biotechnology. 40 (8): 1250–1258. doi:10.1038/s41587-022-01245-x. PMC 9376243. PMID 35332339.
- ^ a b Zhao L, Cao YJ (2019). "Engineered T Cell Therapy for Cancer in the Clinic". Frontiers in Immunology. 10: 2250. doi:10.3389/fimmu.2019.02250. PMC 6798078. PMID 31681259.
- ^ "TCR Vs. CAR-T: What is CAR-T Cell, TCR Therapy, and What are They Used For?". Akadeum Life Sciences. 23 November 2020. Retrieved 14 January 2022.
- ^ a b Yao S, Zhu Y, Chen L (February 2013). "Advances in targeting cell surface signalling molecules for immune modulation". Nature Reviews. Drug Discovery. 12 (2): 130–146. doi:10.1038/nrd3877. PMC 3698571. PMID 23370250.
- ^ a b Scott AM, Wolchok JD, Old LJ (March 2012). "Antibody therapy of cancer". Nature Reviews. Cancer. 12 (4): 278–87. doi:10.1038/nrc3236. PMID 22437872. S2CID 205469234.
- ^ a b Harding FA, Stickler MM, Razo J, DuBridge RB (May–June 2010). "The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions". mAbs. 2 (3): 256–65. doi:10.4161/mabs.2.3.11641. PMC 2881252. PMID 20400861.
- ^ Gadd AJ, Greco F, Cobb AJ, Edwards AD (August 2015). "Targeted Activation of Toll-Like Receptors: Conjugation of a Toll-Like Receptor 7 Agonist to a Monoclonal Antibody Maintains Antigen Binding and Specificity" (PDF). Bioconjugate Chemistry. 26 (8): 1743–52. doi:10.1021/acs.bioconjchem.5b00302. PMID 26133029. S2CID 26307107.
We demonstrate here for the first time the successful conjugation of a small molecule TLR7 agonist to an antitumor mAb (the anti-hCD20 rituximab) without compromising antigen specificity.
- ^ Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, et al. (August 2014). "Type I and type II Fc receptors regulate innate and adaptive immunity". Nature Immunology. 15 (8): 707–16. doi:10.1038/ni.2939. PMC 7430760. PMID 25045879.
- ^ Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. (June 2012). "Safety, activity, and immune correlates of anti-PD-1 antibody in cancer". The New England Journal of Medicine. 366 (26): 2443–54. doi:10.1056/NEJMoa1200690. PMC 3544539. PMID 22658127.
- ^ Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV (October 2015). "FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis". Cancer Cell. 28 (4): 543. doi:10.1016/j.ccell.2015.09.011. PMID 28854351.
- ^ Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, et al. (May 2017). "In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy". Science Translational Medicine. 9 (389): eaal3604. doi:10.1126/scitranslmed.aal3604. PMC 5734617. PMID 28490665.
- ^ Dahan R, Barnhart BC, Li F, Yamniuk AP, Korman AJ, Ravetch JV (July 2016). "Therapeutic Activity of Agonistic, Human Anti-CD40 Monoclonal Antibodies Requires Selective FcγR Engagement". Cancer Cell. 29 (6): 820–31. doi:10.1016/j.ccell.2016.05.001. PMC 4975533. PMID 27265505.
- ^ Weiner LM, Surana R, Wang S (May 2010). "Monoclonal antibodies: versatile platforms for cancer immunotherapy". Nature Reviews. Immunology. 10 (5): 317–27. doi:10.1038/nri2744. PMC 3508064. PMID 20414205.
- ^ Seidel UJ, Schlegel P, Lang P (2013). "Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies". Frontiers in Immunology. 4: 76. doi:10.3389/fimmu.2013.00076. PMC 3608903. PMID 23543707.
- ^ Jaiswal S, Chao MP, Majeti R, Weissman IL (June 2010). "Macrophages as mediators of tumor immunosurveillance". Trends in Immunology. 31 (6): 212–19. doi:10.1016/j.it.2010.04.001. PMC 3646798. PMID 20452821.
- ^ a b c Weiskopf K (May 2017). "Cancer immunotherapy targeting the CD47/SIRPα axis". European Journal of Cancer. 76: 100–09. doi:10.1016/j.ejca.2017.02.013. PMID 28286286.
- ^ Matlung HL, Szilagyi K, Barclay NA, van den Berg TK (March 2017). "The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer". Immunological Reviews. 276 (1): 145–64. doi:10.1111/imr.12527. PMID 28258703. S2CID 6275163.
- ^ Veillette A, Chen J (March 2018). "SIRPα-CD47 Immune Checkpoint Blockade in Anticancer Therapy". Trends in Immunology. 39 (3): 173–84. doi:10.1016/j.it.2017.12.005. PMID 29336991.
- ^ Ahmed M, Cheung NK (January 2014). "Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy". FEBS Letters. 588 (2): 288–97. Bibcode:2014FEBSL.588..288A. doi:10.1016/j.febslet.2013.11.030. PMID 24295643.
- ^ Gelderman KA, Tomlinson S, Ross GD, Gorter A (March 2004). "Complement function in mAb-mediated cancer immunotherapy". Trends in Immunology. 25 (3): 158–64. doi:10.1016/j.it.2004.01.008. PMID 15036044.
- ^ Waldmann TA (March 2003). "Immunotherapy: past, present and future". Nature Medicine. 9 (3): 269–77. doi:10.1038/nm0303-269. PMID 12612576. S2CID 9745527.
- ^ Demko S, Summers J, Keegan P, Pazdur R (February 2008). "FDA drug approval summary: alemtuzumab as single-agent treatment for B-cell chronic lymphocytic leukemia". The Oncologist. 13 (2): 167–74. CiteSeerX 10.1.1.503.6960. doi:10.1634/theoncologist.2007-0218. PMID 18305062.
- ^ "FDA approves new, targeted treatment for bladder cancer". FDA. 18 May 2016. Retrieved 20 May 2016.
- ^ "FDA approves atezolizumab and hyaluronidase-tqjs". U.S. Food and Drug Administration. 12 September 2024. Archived from the original on 14 September 2024. Retrieved 14 September 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "FDA Approves Genentech's Tecentriq Hybreza, the First and Only Subcutaneous Anti-PD-(L)1 Cancer Immunotherapy". Genentech (Press release). 12 September 2024. Archived from the original on 13 September 2024. Retrieved 14 September 2024.
- ^ "Halozyme Announces FDA Approval of Roche's Tecentriq Hybreza With Enhanze for Multiple Types of Cancer" (Press release). Halozyme Therapeutics. 12 September 2024. Archived from the original on 13 September 2024. Retrieved 14 September 2024 – via PR Newswire.
- ^ "US Food and Drug Administration – Avelumab Prescribing Label" (PDF).
- ^ Center for Drug Evaluation and Research. "Approved Drugs – Durvalumab (Imfinzi)". fda.gov. Retrieved 6 May 2017.
- ^ "FDA approves durvalumab after chemoradiation for unresectable stage III NSCLC". FDA. 9 February 2019.
- ^ "Bristol-Myers Squibb and AbbVie Receive U.S. FDA Breakthrough Therapy Designation for Elotuzumab, an Investigational Humanized Monoclonal Antibody for Multiple Myeloma | BMS Newsroom".
- ^ Pazdur R. "FDA approval for Ipilimumab". Archived from the original on 6 April 2015. Retrieved 7 November 2013.
- ^ Sharma P, Allison JP (April 2015). "The future of immune checkpoint therapy". Science. 348 (6230): 56–61. Bibcode:2015Sci...348...56S. doi:10.1126/science.aaa8172. PMID 25838373. S2CID 4608450.
- ^ "Opdivo (nivolumab) FDA Approval History". Drugs.com.
- ^ Lemery SJ, Zhang J, Rothmann MD, Yang J, Earp J, Zhao H, et al. (September 2010). "U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab". Clinical Cancer Research. 16 (17): 4331–38. doi:10.1158/1078-0432.CCR-10-0570. PMID 20601446.
- ^ "FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC". FDA. 20 December 2019.
- ^ "Pembrolizumab (KEYTRUDA) for classical Hodgkin lymphoma". FDA. 9 February 2019.
- ^ "FDA approves pembrolizumab for Merkel cell carcinoma". FDA. 20 December 2019.
- ^ "FDA approves pembrolizumab for treatment of relapsed or refractory PMBCL". FDA. 9 February 2019.
- ^ "National Cancer Institute - Pembrolizumab Use in Cancer". 18 September 2014.
- ^ James JS, Dubs G (December 1997). "FDA approves new kind of lymphoma treatment. Food and Drug Administration". AIDS Treatment News (284): 2–3. PMID 11364912.
- ^ "Rituxan Hycela- rituximab and hyaluronidase injection, solution". DailyMed. 8 July 2024. Retrieved 15 September 2024.
- ^ Byrd JC, Stilgenbauer S, Flinn IW (1 January 2004). "Chronic lymphocytic leukemia". Hematology. American Society of Hematology. Education Program. 2004 (1): 163–83. doi:10.1182/asheducation-2004.1.163. PMID 15561682.
- ^ Domagała A, Kurpisz M (2001). "CD52 antigen--a review". Medical Science Monitor. 7 (2): 325–31. PMID 11257744.
- ^ Dearden C (July 2012). "How I treat prolymphocytic leukemia". Blood. 120 (3): 538–51. doi:10.1182/blood-2012-01-380139. PMID 22649104.
- ^ "Tecentriq- atezolizumab injection, solution". DailyMed. 3 June 2020. Retrieved 31 July 2020.
- ^ "FDA grants approval to atezolizumab for alveolar soft part sarcoma". U.S. Food and Drug Administration (FDA). 9 December 2022. Retrieved 20 December 2022.
- ^ Madhusoodanan J (9 August 2023). "How a controversial US drug policy could be harming cancer patients worldwide". Nature. 620 (7973): 264–267. Bibcode:2023Natur.620..264M. doi:10.1038/d41586-023-02492-x. PMID 37558845. Retrieved 21 August 2023.
- ^ "Genentech Presents Positive Results of Atezolizumab in Advanced Bladder Cancer". 2 October 2015.
- ^ a b "Tecentriq EPAR". European Medicines Agency. 29 September 2017. Retrieved 31 July 2020. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "FDA approves new, targeted treatment for bladder cancer" (Press release). U.S. Food and Drug Administration (FDA). 18 May 2016. Retrieved 20 May 2016.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "European Commission approves Roche's Tecentriq SC, the EU's first PD-(L)1 cancer immunotherapy subcutaneous injection for multiple cancer types – Company Announcement - FT.com". markets.ft.com. Retrieved 5 March 2024.
- ^ a b c d e "Tecentriq Hybreza- atezolizumab and hyaluronidase-tqjs injection". DailyMed. 25 September 2024. Retrieved 5 October 2024.
- ^ a b c "FDA approves atezolizumab and hyaluronidase-tqjs". U.S. Food and Drug Administration. 12 September 2024. Archived from the original on 14 September 2024. Retrieved 14 September 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "FDA Approves Genentech's Tecentriq Hybreza, the First and Only Subcutaneous Anti-PD-(L)1 Cancer Immunotherapy". Genentech (Press release). 12 September 2024. Archived from the original on 13 September 2024. Retrieved 14 September 2024.
- ^ "Halozyme Announces FDA Approval of Roche's Tecentriq Hybreza With Enhanze for Multiple Types of Cancer" (Press release). Halozyme Therapeutics. 12 September 2024. Archived from the original on 13 September 2024. Retrieved 14 September 2024 – via PR Newswire.
- ^ "Bavencio- avelumab injection, solution, concentrate". DailyMed. 2 July 2020. Retrieved 2 August 2020.
- ^ a b c "FDA approves first treatment for rare form of skin cancer". U.S. Food and Drug Administration (FDA) (Press release). 23 March 2017. Retrieved 2 August 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Public summary of opinion on orphan designation: Avelumab for the treatment of gastric cancer" (PDF). European Medicines Agency. 9 January 2017. Archived from the original (PDF) on 5 August 2017. Retrieved 29 April 2017.
- ^ "Bavencio: EPAR - Product Information" (PDF). European Medicines Agency. 18 September 2017. Archived from the original (PDF) on 17 June 2018. Retrieved 23 February 2018.
- ^ Merck-Pfizer Alliance. "Merck-Pfizer Alliance Avelumab Fact Sheet" (PDF). Archived from the original (PDF) on 17 May 2017. Retrieved 2 December 2015.
- ^ "FDA approves durvalumab after chemoradiation for unresectable stage III NSCLC". FDA. 9 February 2019.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2016". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ "Health Canada New Drug Authorizations: 2016 Highlights". Health Canada. 14 March 2017. Retrieved 7 April 2024.
- ^ a b c d e f g "Elotuzumab Package Insert" (PDF). Archived (PDF) from the original on 8 December 2015.
- ^ "Empliciti (elotuzumab) for Injection, for Intravenous Use. Full Prescribing Information" (PDF). Empliciti (elotuzumab) for US Healthcare Professionals. Princeton, New Jersey: Bristol-Myers Squibb Company. Archived from the original (PDF) on 8 December 2015.
- ^ "Bristol Myers Squibb Reports Primary Results of ELOQUENT-1 Study Evaluating Empliciti (elotuzumab) Plus Revlimid (lenalidomide) and Dexamethasone in Patients with Newly Diagnosed, Untreated Multiple Myeloma". news.bms.com. Retrieved 18 March 2021.
- ^ "Bristol-Myers Squibb and AbbVie Receive U.S. FDA Breakthrough Therapy Designation for Elotuzumab, an Investigational Humanized Monoclonal Antibody for Multiple Myeloma" (Press release). Princeton, New Jersey and North Chicago, Illinois: Bristol-Myers Squibb. 19 May 2014. Retrieved 5 February 2015.
- ^ "Bristol-Myers Squibb and AbbVie Receive FDA Approval of Empliciti™ (elotuzumab) for the Treatment of Patients with Multiple Myeloma Who Have Received One to Three Prior Therapies". news.bms.com. Retrieved 18 March 2021.
- ^ BMS gets two new cancer approvals in Europe. May 2016
- ^ "U.S. Food and Drug Administration Approves Empliciti® (elotuzumab) Plus Pomalidomide and Dexamethasone, a New Immunotherapy Combination for Certain Patients with Relapsed or Refractory Multiple Myeloma". news.bms.com. Retrieved 18 March 2021.
- ^ a b Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P (June 2011). "Ipilimumab". Nature Reviews. Drug Discovery. 10 (6): 411–12. doi:10.1038/nrd3463. PMID 21629286.
- ^ a b Lipson EJ, Drake CG (November 2011). "Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma". Clinical Cancer Research. 17 (22): 6958–62. doi:10.1158/1078-0432.CCR-11-1595. PMC 3575079. PMID 21900389.
- ^ a b Thumar JR, Kluger HM (December 2010). "Ipilimumab: a promising immunotherapy for melanoma". Oncology. 24 (14): 1280–88. PMID 21294471.
- ^ a b Chambers CA, Kuhns MS, Egen JG, Allison JP (2001). "CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy". Annual Review of Immunology. 19: 565–94. doi:10.1146/annurev.immunol.19.1.565. PMID 11244047.
- ^ a b Postow MA, Callahan MK, Wolchok JD (June 2015). "Immune Checkpoint Blockade in Cancer Therapy". Journal of Clinical Oncology. 33 (17): 1974–82. doi:10.1200/JCO.2014.59.4358. PMC 4980573. PMID 25605845.
- ^ a b c Pardoll DM (March 2012). "The blockade of immune checkpoints in cancer immunotherapy". Nature Reviews. Cancer. 12 (4): 252–64. doi:10.1038/nrc3239. PMC 4856023. PMID 22437870.
- ^ Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB (2017). "Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy". Frontiers in Pharmacology. 8: 49. doi:10.3389/fphar.2017.00049. PMC 5296331. PMID 28228726.
- ^ Castillo J, Perez K (2010). "The role of ofatumumab in the treatment of chronic lymphocytic leukemia resistant to previous therapies". Journal of Blood Medicine. 1: 1–8. doi:10.2147/jbm.s7284. PMC 3262337. PMID 22282677.
- ^ Zhang B (July–August 2009). "Ofatumumab". mAbs. 1 (4): 326–31. doi:10.4161/mabs.1.4.8895. PMC 2726602. PMID 20068404.
- ^ "Pembrolizumab label" (PDF). FDA. May 2017. linked from Index page at FDA website November 2016
- ^ "Pembrolizumab label at eMC". UK Electronic Medicines Compendium. 27 January 2017. Archived from the original on 13 December 2017. Retrieved 4 October 2018.
- ^ "HIGHLIGHTS OF PRESCRIBING INFORMATION - KEYTRUDA (Pembrolizumab)" (PDF). fda.gov. June 2018. Retrieved 27 February 2019.
- ^ Keating GM (July 2010). "Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma". Drugs. 70 (11): 1445–76. doi:10.2165/11201110-000000000-00000. PMID 20614951.
- ^ Plosker GL, Figgitt DP (2003). "Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia". Drugs. 63 (8): 803–43. doi:10.2165/00003495-200363080-00005. PMID 12662126.
- ^ Cerny T, Borisch B, Introna M, Johnson P, Rose AL (November 2002). "Mechanism of action of rituximab". Anti-Cancer Drugs. 13 (Suppl 2): S3–10. doi:10.1097/00001813-200211002-00002. PMID 12710585. S2CID 25061294.
- ^ Janeway C, Travers P, Walport M, Shlomchik M (2001). Immunobiology (Fifth ed.). New York and London: Garland Science. ISBN 978-0-8153-4101-7.[page needed]
- ^ Weiner GJ (April 2010). "Rituximab: mechanism of action". Seminars in Hematology. 47 (2): 115–23. doi:10.1053/j.seminhematol.2010.01.011. PMC 2848172. PMID 20350658.
- ^ "Trastuzumab - Drugs.com". www.drugs.com. Archived from the original on 7 April 2017. Retrieved 21 December 2016.
- ^ a b "Kanjinti- trastuzumab-anns injection, powder, lyophilized, for solution; Kanjinti- trastuzumab-anns kit". DailyMed. 13 October 2022. Archived from the original on 5 December 2021. Retrieved 11 December 2023.
- ^ a b "Ogivri- trastuzumab kit; Ogivri- trastuzumab injection, powder, lyophilized, for solution". DailyMed. 8 February 2021. Archived from the original on 7 December 2022. Retrieved 11 December 2023.
- ^ a b "Ontruzant- trastuzumab injection, powder, lyophilized, for solution; Ontruzant- ontruzant kit". DailyMed. 31 January 2023. Archived from the original on 13 August 2022. Retrieved 11 December 2023.
- ^ a b "Trazimera- trastuzumab-qyyp kit; Trazimera- trastuzumab-qyyp injection, powder, lyophilized, for solution". DailyMed. 14 February 2022. Archived from the original on 7 December 2022. Retrieved 11 December 2023.
- ^ a b "Highlights of prescribing information" (PDF). www.accessdata.fda.gov.
- ^ "Herzuma- trastuzumab kit; Herzuma- trastuzumab injection, powder, lyophilized, for solution". DailyMed. 6 February 2023. Archived from the original on 16 May 2021. Retrieved 11 December 2023.
- ^ "Herzuma EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 8 November 2020. Retrieved 28 July 2020.
- ^ "Herzuma PI". Union Register of medicinal products. 13 February 2018. Retrieved 30 September 2024.
- ^ "Herwenda EPAR". European Medicines Agency. 15 November 2023. Archived from the original on 3 December 2023. Retrieved 11 December 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Herwenda Product information". Union Register of medicinal products. 16 November 2023. Archived from the original on 26 November 2023. Retrieved 11 December 2023.
- ^ "Kanjinti EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 31 October 2020. Retrieved 28 July 2020.
- ^ "Kanjinti PI". Union Register of medicinal products. 18 May 2018. Retrieved 30 September 2024.
- ^ "Ogivri- trastuzumab kit; Ogivri- trastuzumab injection, powder, lyophilized, for solution". DailyMed. 28 July 2023. Archived from the original on 10 March 2024. Retrieved 11 December 2023.
- ^ "Ontruzant EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 4 August 2020. Retrieved 28 July 2020.
- ^ "Ontruzant PI". Union Register of medicinal products. 17 November 2017. Retrieved 30 September 2024.
- ^ a b c d "Trastucip and Tuzucip APMDS". Therapeutic Goods Administration (TGA). 29 July 2022. Archived from the original on 30 July 2022. Retrieved 2 August 2022.
- ^ "Tuznue EPAR". European Medicines Agency. 26 July 2024. Retrieved 29 July 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Tuznue PI". Union Register of medicinal products. 24 September 2024. Retrieved 27 September 2024.
- ^ "Zercepac EPAR". European Medicines Agency (EMA). 26 May 2020. Archived from the original on 28 July 2020. Retrieved 28 July 2020.
- ^ "Zercepac PI". Union Register of medicinal products. 28 July 2020. Retrieved 30 September 2024.
- ^ a b "Trastuzumab Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 3 December 2019. Retrieved 3 December 2019.
- ^ "Herceptin (trastuzumab) powder for injection". Roche Products Pty Limited. 7 May 2021. Archived from the original on 8 March 2023. Retrieved 8 January 2023.
- ^ "Trastuzumab". Roche Products Pty Limited. 7 April 2022. Archived from the original on 8 January 2023.
- ^ "Summary Basis of Decision - Ontruzant". Health Canada. 23 October 2014. Archived from the original on 6 August 2022. Retrieved 6 August 2022.
- ^ a b "Herceptin- trastuzumab kit Herceptin- trastuzumab injection, powder, lyophilized, for solution". DailyMed. 30 September 2019. Archived from the original on 4 August 2020. Retrieved 28 July 2020.
- ^ "Ogivri EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 24 March 2020. Retrieved 28 July 2020.
- ^ "Ogivri PI". Union Register of medicinal products. 14 December 2018. Retrieved 30 September 2024.
- ^ a b c d e f g "Trastuzumab". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ "FDA approves first biosimilar for the treatment of certain breast and stomach cancers". U.S. Food and Drug Administration (FDA) (Press release). 10 September 2019. Archived from the original on 15 December 2019. Retrieved 18 February 2020.
- ^ a b "Herceptin EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 28 July 2020. Retrieved 28 July 2020.
- ^ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 626. ISBN 978-0-85711-156-2.
- ^ "Trastuzumab Product Approval Information - Licensing Action 9/25/98". U.S. Food and Drug Administration (FDA). 18 December 2015. Archived from the original on 28 January 2017. Retrieved 3 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ Ganesan S, Mehnert J (9 March 2020). "Biomarkers for Response to Immune Checkpoint Blockade". Annual Review of Cancer Biology. 4 (1): 331–351. doi:10.1146/annurev-cancerbio-030419-033604.
- ^ Magahis PT, Maron SB, Cowzer D, King S, Schattner M, Janjigian Y, et al. (October 2023). "Impact of Helicobacter pylori infection status on outcomes among patients with advanced gastric cancer treated with immune checkpoint inhibitors". J Immunother Cancer. 11 (10): e007699. doi:10.1136/jitc-2023-007699. PMC 10619027. PMID 37899129.
- ^ Yu B, Peppelenbosch M, Fuhler G (January 2024). "Impact of Helicobacter pylori infection status on outcomes among patients with advanced gastric cancer treated with immune checkpoint inhibitors". J Immunother Cancer. 12 (1): e008422. doi:10.1136/jitc-2023-008422. PMC 10806497. PMID 38242721.
- ^ Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E, et al. (2017). "Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer". ESMO Open. 2 (2): e000213. doi:10.1136/esmoopen-2017-000213. PMC 5518304. PMID 28761757.
- ^ Cameron F, Whiteside G, Perry C (May 2011). "Ipilimumab: first global approval". Drugs. 71 (8): 1093–104. doi:10.2165/11594010-000000000-00000. PMID 21668044.
- ^ Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. (June 2012). "Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study". Journal of Clinical Oncology. 30 (17): 2046–54. doi:10.1200/JCO.2011.38.4032. PMID 22547592.
- ^ Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. (September 2013). "Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer". Journal of Immunotherapy. 36 (7): 382–89. doi:10.1097/CJI.0b013e31829fb7a2. PMC 3779664. PMID 23924790.
- ^ Clinical trial number NCT01928394 for "A Study of Nivolumab by Itself or Nivolumab Combined With Ipilimumab in Patients With Advanced or Metastatic Solid Tumors" at ClinicalTrials.gov
- ^ Gougis P, Hamy AS, Jochum F, Bihan K, Carbonnel M, Salem JE, et al. (April 2024). "Immune Checkpoint Inhibitor Use During Pregnancy and Outcomes in Pregnant Individuals and Newborns". JAMA Network Open. 7 (4): e245625. doi:10.1001/jamanetworkopen.2024.5625. PMC 11024778. PMID 38630478.
- ^ a b van Hooren L, Sandin LC, Moskalev I, Ellmark P, Dimberg A, Black P, et al. (February 2017). "Local checkpoint inhibition of CTLA-4 as a monotherapy or in combination with anti-PD1 prevents the growth of murine bladder cancer". European Journal of Immunology. 47 (2): 385–93. doi:10.1002/eji.201646583. PMID 27873300. S2CID 2463514.
- ^ a b Pollack A (18 May 2016). "F.D.A. Approves an Immunotherapy Drug for Bladder Cancer". The New York Times. ISSN 0362-4331. Retrieved 21 May 2016.
- ^ Steele A (5 August 2016). "Bristol Myers: Opdivo Failed to Meet Endpoint in Key Lung-Cancer Study". The Wall Street Journal. ISSN 0099-9660. Retrieved 5 August 2016.
- ^ BeiGene, Ltd. (2016). "BeiGene Presents Initial Clinical Data on PD-1 Antibody BGB-A317 at the 2016 American Society of Clinical Oncology Annual Meeting" (Press release). Globe Newswire.
- ^ Roche. "FDA grants priority review for Roche's cancer immunotherapy atezolizumab in specific type of lung cancer".
- ^ Merck Group. "Immuno-oncology Avelumab".
- ^ Cure today (April 2016). "Durvalumab continues to progress in treatment of advanced bladder cancer".
- ^ Avacta Life Sciences. "Affimer biotherapeutics target cancer's off-switch with PD-L1 inhibitor". Archived from the original on 6 August 2016. Retrieved 16 May 2016.
- ^ Palmer DC, Guittard GC, Franco Z, Crompton JG, Eil RL, Patel SJ, et al. (November 2015). "Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance". The Journal of Experimental Medicine. 212 (12): 2095–2113. doi:10.1084/jem.20150304. PMC 4647263. PMID 26527801.
- ^ Guittard G, Dios-Esponera A, Palmer DC, Akpan I, Barr VA, Manna A, et al. (March 2018). "The Cish SH2 domain is essential for PLC-γ1 regulation in TCR stimulated CD8+ T cells". Scientific Reports. 8 (1): 5336. Bibcode:2018NatSR...8.5336G. doi:10.1038/s41598-018-23549-2. PMC 5871872. PMID 29593227.
- ^ Palmer DC, Guittard GC, Franco Z, Crompton JG, Eil RL, Patel SJ, et al. (November 2015). "Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance". The Journal of Experimental Medicine. 212 (12): 2095–2113. doi:10.1084/jem.20150304. PMC 4647263. PMID 26527801.
- ^ Palmer DC, Webber BR, Patel Y, Johnson MJ, Kariya CM, Lahr WS, et al. (25 September 2020). "Internal checkpoint regulates T cell neoantigen reactivity and susceptibility to PD1 blockade". bioRxiv 10.1101/2020.09.24.306571.
- ^ Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. (February 2016). "Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy". Immunity. 44 (2): 343–54. doi:10.1016/j.immuni.2015.11.024. PMC 4758865. PMID 26872698.
- ^ Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD (2017). "Combination immunotherapy: a road map". Journal for Immunotherapy of Cancer. 5: 16. doi:10.1186/s40425-017-0218-5. PMC 5319100. PMID 28239469.
- ^ Mahoney KM, Rennert PD, Freeman GJ (August 2015). "Combination cancer immunotherapy and new immunomodulatory targets". Nature Reviews. Drug Discovery. 14 (8): 561–84. doi:10.1038/nrd4591. PMID 26228759. S2CID 2220735.
- ^ Mehta A, Oklu R, Sheth RA (2015). "Thermal Ablative Therapies and Immune Checkpoint Modulation: Can Locoregional Approaches Effect a Systemic Response?". Gastroenterology Research and Practice. 2016: 9251375. doi:10.1155/2016/9251375. PMC 4802022. PMID 27051417.
- ^ Tang J, Shalabi A, Hubbard-Lucey VM (January 2018). "Comprehensive analysis of the clinical immuno-oncology landscape". Annals of Oncology. 29 (1): 84–91. doi:10.1093/annonc/mdx755. PMID 29228097.
- ^ Perry CJ, Muñoz-Rojas AR, Meeth KM, Kellman LN, Amezquita RA, Thakral D, et al. (March 2018). "Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity". The Journal of Experimental Medicine. 215 (3): 877–93. doi:10.1084/jem.20171435. PMC 5839759. PMID 29436395.
- ^ Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, et al. (21 May 2018). "TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy". Nature Biomedical Engineering. 2 (8): 578–588. doi:10.1038/s41551-018-0236-8. PMC 6192054. PMID 31015631.
- ^ Zak J, Pratumchai I, Marro BS, Marquardt KL, Zavareh RB, Lairson LL, et al. (June 2024). "JAK inhibition enhances checkpoint blockade immunotherapy in patients with Hodgkin lymphoma". Science. 384 (6702): eade8520. doi:10.1126/science.ade8520. PMC 11283877. PMID 38900864.
- ^ a b Dranoff G (January 2004). "Cytokines in cancer pathogenesis and cancer therapy". Nature Reviews. Cancer. 4 (1): 11–22. doi:10.1038/nrc1252. PMID 14708024. S2CID 42092046.
- ^ "Immunotherapy For Cancer". Retrieved 12 May 2023.
- ^ Dunn GP, Koebel CM, Schreiber RD (November 2006). "Interferons, immunity and cancer immunoediting". Nature Reviews. Immunology. 6 (11): 836–48. doi:10.1038/nri1961. PMID 17063185. S2CID 223082.
- ^ Lasfar A, Abushahba W, Balan M, Cohen-Solal KA (2011). "Interferon lambda: a new sword in cancer immunotherapy". Clinical & Developmental Immunology. 2011: 349575. doi:10.1155/2011/349575. PMC 3235441. PMID 22190970.
- ^ Razaghi A, Owens L, Heimann K (December 2016). "Review of the recombinant human interferon gamma as an immunotherapeutic: Impacts of production platforms and glycosylation". Journal of Biotechnology. 240: 48–60. doi:10.1016/j.jbiotec.2016.10.022. PMID 27794496.
- ^ Coventry BJ, Ashdown ML (2012). "The 20th anniversary of interleukin-2 therapy: bimodal role explaining longstanding random induction of complete clinical responses". Cancer Management and Research. 4: 215–21. doi:10.2147/cmar.s33979. PMC 3421468. PMID 22904643.
- ^ "FDA approves Encorafenib and Binimetinib in combination for unresectable or metastatic melanoma with BRAF mutations". U.S. Food and Drug Administration. 27 June 2018.
- ^ "Cancer Genetics offers the FDA-approved DAKO PD-L1 IHC 22C3 pharmDx companion diagnostic test for KEYTRUDA®". 3 February 2016.
- ^ Udall M, Rizzo M, Kenny J, Doherty J, Dahm S, Robbins P, et al. (February 2018). "PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics". Diagnostic Pathology. 13 (1): 12. doi:10.1186/s13000-018-0689-9. PMC 5807740. PMID 29426340.
- ^ Dacic S (April 2018). "Time is up for PD-L1 testing standardization in lung cancer". Annals of Oncology. 29 (4): 791–792. doi:10.1093/annonc/mdy069. PMID 29688334.
- ^ Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. (November 2017). "Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers". Molecular Cancer Therapeutics. 16 (11): 2598–2608. doi:10.1158/1535-7163.MCT-17-0386. PMC 5670009. PMID 28835386.
- ^ "FDA Accepts sBLA for First-Line Nivolumab Plus Low-Dose Ipilimumab in NSCLC With Tumor Mutational Burden ≥ 10 mut/mb". ASCO Post. American Society of Clinical Oncology. 7 February 2018.
- ^ Liu D, Schilling B, Liu D, Sucker A, Livingstone E, Jerby-Arnon L, et al. (December 2019). "Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma". Nature Medicine. 25 (12): 1916–1927. doi:10.1038/s41591-019-0654-5. PMC 6898788. PMID 31792460.
- ^ Motzer RJ, Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, et al. (September 2020). "Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial". Nature Medicine. 26 (11): 1733–1741. doi:10.1038/s41591-020-1044-8. PMC 8493486. PMID 32895571.
- ^ Flam F (22 January 2015). "Duke U Cancer Fraud Scandal: A Cautionary Tale For Obama's Precision Medicine Push". Forbes. Retrieved 21 April 2024.
- ^ "Liquid biopsies" for cancer screening: Life-saving tests, or overdiagnosis and overtreatment taken to a new level? David Gorski, 28 September 2015, Science-Based Medicine website
- ^ A public discussion by cancer patients from 2011 on the melanoma.org website shows costs and claims.
- ^ Fukuhara H, Ino Y, Todo T (October 2016). "Oncolytic virus therapy: A new era of cancer treatment at dawn". Cancer Science. 107 (10): 1373–79. doi:10.1111/cas.13027. PMC 5084676. PMID 27486853.
- ^ Haddad D (2017). "Genetically Engineered Vaccinia Viruses As Agents for Cancer Treatment, Imaging, and Transgene Delivery". Frontiers in Oncology. 7: 96. doi:10.3389/fonc.2017.00096. PMC 5440573. PMID 28589082.
- ^ Marin-Acevedo JA, Soyano AE, Dholaria B, Knutson KL, Lou Y (January 2018). "Cancer immunotherapy beyond immune checkpoint inhibitors". Journal of Hematology & Oncology. 11 (1): 8. doi:10.1186/s13045-017-0552-6. PMC 5767051. PMID 29329556.
- ^ Lawler SE, Speranza MC, Cho CF, Chiocca EA (June 2017). "Oncolytic Viruses in Cancer Treatment: A Review". JAMA Oncology. 3 (6): 841–849. doi:10.1001/jamaoncol.2016.2064. PMID 27441411. S2CID 39321536.
- ^ Aleem E (June 2013). "β-Glucans and their applications in cancer therapy: focus on human studies". Anti-Cancer Agents in Medicinal Chemistry. 13 (5): 709–19. doi:10.2174/1871520611313050007. PMID 23293888.
- ^ Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. (December 2014). "Genetic basis for clinical response to CTLA-4 blockade in melanoma". The New England Journal of Medicine. 371 (23): 2189–99. doi:10.1056/NEJMoa1406498. PMC 4315319. PMID 25409260.
- ^ Schumacher TN, Schreiber RD (April 2015). "Neoantigens in cancer immunotherapy". Science. 348 (6230): 69–74. Bibcode:2015Sci...348...69S. doi:10.1126/science.aaa4971. PMID 25838375.
- ^ "Coriolus Versicolor". American Cancer Society. Archived from the original on 15 February 2006.
External links
[edit]- A primer on "Immunotherapy to Treat Cancer", NIH
- Immunotherapy – Using the Immune System to Treat Cancer Archived 4 April 2017 at the Wayback Machine
- Cancer Research Institute – What is Cancer Immunotherapy
- Society for Immunotherapy of Cancer
- "And Then There Were Five". Economist.
- "Discover the Science of Immuno-Oncology". Bristol-Myers Squibb. Archived from the original on 10 October 2014. Retrieved 13 March 2014.
- Eggermont A, Finn O (September 2012). "Advances in immuno-oncology. Foreword". Annals of Oncology. 23 (Suppl 8): viii5. doi:10.1093/annonc/mds255. PMID 22918929.
- "Cancer Immunotherapy in Gujarat"
