Cyclopropane
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclopropane[2] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.771 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 1027 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6 | |||
| Molar mass | 42.08 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Sweet, ethereal | ||
| Density | 1.879 g/L (1 atm, 0 °C) 680 g/L (liquid) | ||
| Melting point | −128 °C (−198 °F; 145 K) | ||
| Boiling point | −32.9 °C (−27.2 °F; 240.2 K) | ||
| 502 mg/L | |||
| Vapor pressure | 640 kPa (20 °C) 1350 kPa (50 °C) | ||
| Acidity (pKa) | ~46 | ||
| -39.9·10−6 cm3/mol | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Highly flammable Asphyxiant | ||
| GHS labelling: | |||

| |||
| Danger | |||
| NFPA 704 (fire diamond) | |||
| 495 °C (923 °F; 768 K) | |||
| Explosive limits | 2.4 % (lower) 10.4 % (upper) | ||
| Safety data sheet (SDS) | Air Liquide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
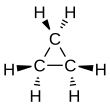
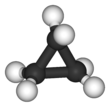
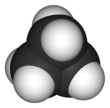
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives - cyclopropanes - are of commercial or biological significance.[3]
Cyclopropane was used as a clinical inhalational anesthetic from the 1930s through the 1980s. The substance's high flammability poses a risk of fire and explosions in operating rooms due to its tendency to accumulate in confined spaces, as its density is higher than that of air.
History
[edit]Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper.[4] Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane.[5] The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium.[6] Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929;[7] industrial production had begun by 1936.[8] In modern anaesthetic practice, it has been superseded by other agents.
Anaesthesia
[edit]Cyclopropane was introduced into clinical use by the American anaesthetist Ralph Waters who used a closed system with carbon dioxide absorption to conserve this then-costly agent. Cyclopropane is a relatively potent, non-irritating and sweet smelling agent with a minimum alveolar concentration of 17.5%[9] and a blood/gas partition coefficient of 0.55. This meant induction of anaesthesia by inhalation of cyclopropane and oxygen was rapid and not unpleasant. However at the conclusion of prolonged anaesthesia patients could suffer a sudden decrease in blood pressure, potentially leading to cardiac dysrhythmia: a reaction known as "cyclopropane shock".[10] For this reason, as well as its high cost and its explosive nature,[11] it was latterly used only for the induction of anaesthesia, and has not been available for clinical use since the mid-1980s. Cylinders and flow meters were colored orange.
Pharmacology
[edit]Cyclopropane is inactive at the GABAA and glycine receptors, and instead acts as an NMDA receptor antagonist.[12][13] It also inhibits the AMPA receptor and nicotinic acetylcholine receptors, and activates certain K2P channels.[12][13][14]
Structure and bonding
[edit]
The triangular structure of cyclopropane requires the bond angles between carbon-carbon covalent bonds to be 60°. The molecule has D3h molecular symmetry. The C-C distances are 151 pm versus 153-155 pm.[15][16]
Despite their shortness, the C-C bonds in cyclopropane are weakened by 34 kcal/mol vs ordinary C-C bonds. In addition to ring strain, the molecule also has torsional strain due to the eclipsed conformation of its hydrogen atoms. The C-H bonds in cyclopropane are stronger than ordinary C-H bonds as reflected by NMR coupling constants.
Bonding between the carbon centres is generally described in terms of bent bonds.[17] In this model the carbon-carbon bonds are bent outwards so that the inter-orbital angle is 104°.
The unusual structural properties of cyclopropane have spawned many theoretical discussions. One theory invokes σ-aromaticity: the stabilization afforded by delocalization of the six electrons of cyclopropane's three C-C σ bonds to explain why the strain of cyclopropane is "only" 27.6 kcal/mol as compared to cyclobutane (26.2 kcal/mol) with cyclohexane as reference with Estr=0 kcal/mol,[18][19][20] in contrast to the usual π aromaticity, that, for example, has a highly stabilizing effect in benzene. Other studies do not support the role of σ-aromaticity in cyclopropane and the existence of an induced ring current; such studies provide an alternative explanation for the energetic stabilization and abnormal magnetic behaviour of cyclopropane.[21]
Synthesis
[edit]Cyclopropane was first produced via a Wurtz coupling, in which 1,3-dibromopropane was cyclised using sodium.[4] The yield of this reaction can be improved by the use of zinc as the dehalogenating agent and sodium iodide as a catalyst.[22]
- BrCH2CH2CH2Br + 2 Na → (CH2)3 + 2 NaBr
The preparation of cyclopropane rings is referred to as cyclopropanation.
Reactions
[edit]Owing to the increased π-character of its C-C bonds, cyclopropane is often assumed to add bromine to give 1,3-dibromopropane, but this reaction proceeds poorly.[23] Hydrohalogenation with hydrohalic acids gives linear 1-halopropanes. Substituted cyclopropanes also react, following Markovnikov's rule.[24]
Cyclopropane and its derivatives can oxidatively add to transition metals, in a process referred to as C–C activation.
Safety
[edit]Cyclopropane is highly flammable. However, despite its strain energy it does not exhibit explosive behavior substantially different from other alkanes.
See also
[edit]- Tetrahedrane contains four fused cyclopropane rings that form the faces of a tetrahedron
- Propellane contains three cyclopropane rings that share a single central carbon-carbon bond.
- Spiropentane is two cyclopropane rings fused at a vertex
- Cyclopropene
- Methylenecyclopropane
References
[edit]- ^ Merck Index, 11th Edition, 2755.
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 137. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Faust, Rüdiger (2001). "Fascinating Natural and Artificial Cyclopropane Architectures". Angewandte Chemie International Edition. 40 (12): 2251–2253. doi:10.1002/1521-3773(20010618)40:12<2251::AID-ANIE2251>3.0.CO;2-R. PMID 11433485.
- ^ a b August Freund (1881). "Über Trimethylen" [On trimethylene]. Journal für Praktische Chemie. 26 (1): 367–377. doi:10.1002/prac.18820260125.
- ^ August Freund (1882). "Über Trimethylen" [On trimethylene]. Monatshefte für Chemie. 3 (1): 625–635. doi:10.1007/BF01516828. S2CID 197767176.
- ^ G. Gustavson (1887). "Ueber eine neue Darstellungsmethode des Trimethylens" [On a new method of preparing trimethylene]. Journal für Praktische Chemie. 36: 300–305. doi:10.1002/prac.18870360127.
- ^ G. H. W. Lucas; V. E. Henderson (1 August 1929). "A New Anesthetic: Cyclopropane : A Preliminary Report". Can Med Assoc J. 21 (2): 173–5. PMC 1710967. PMID 20317448.
- ^ H. B. Hass; E. T. McBee; G. E. Hinds (1936). "Synthesis of Cyclopropane". Industrial & Engineering Chemistry. 28 (10): 1178–81. doi:10.1021/ie50322a013.
- ^ Eger, Edmond I.; Brandstater, Bernard; Saidman, Lawrence J.; Regan, Michael J.; Severinghaus, John W.; Munson, Edwin S. (1965). "Equipotent Alveolar Concentrations of Methoxyflurane, Halothane, Diethyl Ether, Fluroxene, Cyclopropane, Xenon and Nitrous Oxide in the Dog". Anesthesiology. 26 (6): 771–777. doi:10.1097/00000542-196511000-00012. PMID 4378907.
- ^ JOHNSTONE, M; Alberts, JR (July 1950). "Cyclopropane anesthesia and ventricular arrhythmias". British Heart Journal. 12 (3): 239–44. doi:10.1136/hrt.12.3.239. PMC 479392. PMID 15426685.
- ^ MacDonald, AG (June 1994). "A short history of fires and explosions caused by anaesthetic agents". British Journal of Anaesthesia. 72 (6): 710–22. doi:10.1093/bja/72.6.710. PMID 8024925.
- ^ a b Hugh C. Hemmings; Philip M. Hopkins (2006). Foundations of Anesthesia: Basic Sciences for Clinical Practice. Elsevier Health Sciences. pp. 292–. ISBN 978-0-323-03707-5.
- ^ a b Hemmings, Hugh C. (2009). "Molecular Targets of General Anesthetics in the Nervous System". Suppressing the Mind. Contemporary Clinical Neuroscience. pp. 11–31. doi:10.1007/978-1-60761-462-3_2. ISBN 978-1-60761-463-0.
- ^ Hara K, Eger EI, Laster MJ, Harris RA (December 2002). "Nonhalogenated alkanes cyclopropane and butane affect neurotransmitter-gated ion channel and G-protein-coupled receptors: differential actions on GABAA and glycine receptors". Anesthesiology. 97 (6): 1512–20. doi:10.1097/00000542-200212000-00025. PMID 12459679. S2CID 21160239.[permanent dead link]
- ^ Allen, Frank H.; Kennard, Olga; Watson, David G.; Brammer, Lee; Orpen, A. Guy; Taylor, Robin (1987). "Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds". Journal of the Chemical Society, Perkin Transactions 2 (12): S1 – S19. doi:10.1039/P298700000S1.
- ^ Boulatov, Roman, ed. (2015). Polymer Mechanochemistry. Springer. p. 9. ISBN 978-3-319-22824-2.
- ^ Eric V. Anslyn and Dennis A. Dougherty. Modern Physical Organic Chemistry. 2006. pages 850-852
- ^ S. W. Benson, Thermochemical Kinetics, S. 273, J. Wiley & Sons, New York, London, Sydney, Toronto 1976
- ^ Dewar, M. J. (1984). "Chemical Implications of σ Conjugation". J. Am. Chem. Soc. 106 (3): 669–682. doi:10.1021/ja00315a036.
- ^ Cremer, D. (1988). "Pros and Cons of σ-Aromaticity". Tetrahedron. 44 (2): 7427–7454. doi:10.1016/s0040-4020(01)86238-4.
- ^ Wu, Wei; Ma, Ben; Wu, Judy I-Chia; von Ragué, Schleyer; Mo, Yirong (2009). "Is Cyclopropane Really the σ-Aromatic Paradigm?". Chemistry: A European Journal. 15 (38): 9730–9736. doi:10.1002/chem.200900586. PMID 19562784.
- ^ Wollweber, Hartmund (2000). "Anesthetics, General". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_289. ISBN 978-3527306732.
- ^ Gordon, Arnold J. (1967). "Halogenation and olefinic nature of cyclopropane". Journal of Chemical Education. 44 (8): 461. doi:10.1021/ed044p461.
- ^ Advanced organic Chemistry, Reactions, mechanisms and structure 3ed. Jerry March ISBN 0-471-85472-7