Dienone

A dienone is a class of organic compounds with the general formula (R2C=CR)2C=O, where R is any substituent, but often H. They are formally "derived from 1,4-diene compounds by conversion of a –CH2– groups into –C(=O)– group .", resulting in "a conjugated structure". They are a kind of enone. The class includes some heterocyclic compounds.
Properties and occurrence
[edit]There is no evidence that phenols tautomerizes to a dienone, but such tautomerism is well recognized for resorcinols and some hydroxypyridines.
Being multifunctional, dienones engage in many reactions. They are often good dienophiles. They function as ligands, forming metal-alkene complexes such as tris(dibenzylideneacetone)dipalladium(0).
Cyclic dienones
[edit]Extensive work has been reported on cyclic dienones. The parent of the seven-membered ring series is tropone. It is a not only a dienone but a trieneone.
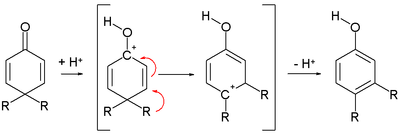
Cyclohexadienones are a significant class of dienones, the premier members being the ortho- and para-quinones. Many cyclohexadienones convert to phenols.[1] In the dienone–phenol rearrangement, they convert to phenols[2] through the dienone–phenol rearrangement:[3]
The parent cyclopentadienone has only a fleeting existence under laboratory conditions, otherwise it dimerizes. The substituted derivative tetraphenylcyclopentadienone is however robust solid.[4]
See also
[edit]References
[edit]- ^ Wipf, Peter; Jung, Jae-Kyu (1999). "Nucleophilic Additions to 4,4-Disubstituted 2,5-Cyclohexadienones: Can Dipole Effects Control Facial Selectivity?". Chemical Reviews. 99 (5): 1469–1480. doi:10.1021/cr9803838. PMID 11749453.
- ^ Related to quinones, see for example the Zincke-Suhl reaction
- ^ Advanced organic Chemistry, Reactions, mechanisms and structure 3ed. page Jerry March ISBN 0-471-85472-7
- ^ Ogliaruso, Michael A.; Romanelli, Michael G.; Becker, Ernest I. (1965). "Chemistry of Cyclopentadienones". Chemical Reviews. 65 (3): 261–367. doi:10.1021/cr60235a001.