Copper IUD
| Copper IUD | |
|---|---|
 Photo of a common IUD (Paragard T 380A) | |
| Background | |
| Type | Intrauterine |
| First use | 1970s[1] |
| Trade names | copper-T, ParaGard, others |
| AHFS/Drugs.com | FDA Professional Drug Information |
| Failure rates (first year) | |
| Perfect use | 0.6%[2] |
| Typical use | 0.8%[2] |
| Usage | |
| Duration effect | 5–12+ years[1] |
| Reversibility | rapid[1] |
| User reminders | Check thread position after each period. Have removed shortly after menopause, if not before. |
| Clinic review | Annually |
| Advantages and disadvantages | |
| STI protection | No |
| Periods | May be heavier and more painful[3] |
| Benefits | Unnecessary to take any daily action. Emergency contraception if inserted within 5 days |
| Risks | <1 in 100: pelvic infection within 20 days of insertion 1.1 in 1000: uterine perforation |
A copper intrauterine device (IUD), also known as an intrauterine coil or copper coil or non-hormonal IUD, is a type of intrauterine device which contains copper.[3] It is used for birth control and emergency contraception within five days of unprotected sex.[3] It is one of the most effective forms of birth control with a one-year failure rate of 0.6-0.8%, and a ten-year failure rate of 1.9%[4][5] The device is placed in the uterus and lasts up to twelve years, depending on the amount of copper present in the device.[3][1][6] It may be used for contraception regardless of age or previous pregnancy.[7] Following removal, fertility quickly returns.[1]
Common side effects include heavy menstrual periods. Rarely the device may come out.[3] It is less recommended for people at high risk of sexually transmitted infections as it may increase the risk of pelvic inflammatory disease in the first three weeks after insertion.[7] It is recommended for people who don't tolerate or hardly tolerate hormonal contraceptives. If someone becomes pregnant with an IUD in place, removal is recommended.[7] Very rarely, uterine perforation may occur during insertion, or the device may become embedded into the uterine wall.[1] The copper IUD is a type of long-acting reversible birth control.[4] It primarily works by killing the sperm.[1]
The copper IUD came into medical use in the 1970s.[1] It is on the World Health Organization's List of Essential Medicines.[8] They are estimated to be used by more than 170 million people globally.[9][10]
Medical uses
[edit]Copper IUDs are a form of long-acting reversible contraception and are one of the most effective forms of birth control available.[11] The type of frame and amount of copper can affect the effectiveness of different copper IUD models.[12] The failure rates for different models vary between 0.1 and 2.2% after 1 year of use. The T-shaped models with a surface area of 380 mm2 of copper have the lowest failure rates. The TCu 380A (ParaGard) has a one-year failure rate of 0.8% and a cumulative 12-year failure rate of 2.2%.[12] Over 12 years of use, the models with less surface area of copper have higher failure rates. The TCu 220A has a 12-year failure rate of 5.8%. The frameless GyneFix has a failure rate of less than 1% per year.[13] Worldwide, older IUD models with lower effectiveness rates are no longer produced.[14]
A 2008 review of the available T-shaped copper IUDs recommended that the TCu 380A and the TCu 280S be used as the first choice for copper IUDs because those two models have the lowest failure rates and the longest lifespans.[12] The effectiveness of the copper IUD (failure rate of 0.8%) is comparable to tubal sterilization (failure rate of 0.5%) for the first year.[15][16][17]
The copper IUD is effective as contraception as soon as it is inserted and loses efficacy when removed or if it becomes malpositioned.[5] Though only approved by regulatory agencies for a maximum of 12 years, it may be effective with continuous use for up to 20 years.[18]
Emergency contraception
[edit]It was first discovered in 1976 that the copper IUD could be used as a form of emergency contraception (EC).[19] The copper IUD is the most effective form of emergency contraception. It is more effective than the hormonal EC pills currently available.[20] Efficacy is not affected by weight.[5] The pregnancy rate among those using the copper IUD for EC is 0.09%. It can be used for EC up to five days after the act of unprotected sex and does not decrease in effectiveness during the five days.[21] An additional advantage of using the copper IUD for emergency contraception is that it can be used as a form of birth control for 10–12 years after insertion.[21]
Removal and return to fertility
[edit]Removal of the copper IUD should also be performed by a qualified medical practitioner. Fertility has been shown to return to previous levels quickly after removal of the device.[22] One study found that the median amount of time from removal to planned pregnancy was three months for those women using the TCu 380Ag.[23]
Side effects and complications
[edit]Complications
[edit]The most common complications related to the copper IUD are expulsion, perforation, and infection. Infertility after discontinuation and difficulty breastfeeding during use are not associated with the copper IUD.[5][22]
Expulsion rates can range from 2.2% to 11.4% of users from the first year to the 10th year. The TCu380A may have lower rates of expulsion than other models.[24] Unusual vaginal discharge, cramping or pain, spotting between periods, postcoital (after sex) spotting, dyspareunia, or the absence or lengthening of the strings can be signs of a possible expulsion.[22] If expulsion occurs, the woman is not protected against pregnancy. If an IUD with copper is inserted after an expulsion has occurred, the risk of re-expulsion has been estimated in one study to be approximately one third of cases after one year.[25] Magnetic resonance imaging (MRI) may cause dislocation of a copper IUD, and it is therefore recommended to check the location of the IUD both before and after MRI.[26]

Perforation of the device through the uterine wall typically occurs at the time of placement, though it may occur spontaneously during the period of use. Approximately 1.1 per 1000 copper IUD insertions are complicated by perforation.[5]
The insertion of a copper IUD poses a transient risk of pelvic inflammatory disease (PID) for 21 days, though this is almost always in the setting of undiagnosed gonorrhea or chlamydia infection at the time of insertion. This occurs in less than 1 in 100 insertions. Beyond this timeframe there is no increased risk of PID associated with copper IUD use.[18][27][5][28][22]
Side effects
[edit]The most common side effects reported with use of the copper IUD are increased menstrual bleeding (20-50% volume increase) and menstrual cramps, both of which may remit after 3-6 months of use. Less frequently, intermenstrual bleeding may occur, especially in the first 3-6 months of use.[5][22]
Menorrhagia (increased menstrual bleeding) and dysmenorrhea (painful menstrual bleeding) are typically treated with NSAID medications, including naproxen, ibuprofen, and mefenamic acid.[29][18]
Contraceptive failure
[edit]
The absolute risk of ectopic pregnancy with IUD use is lower than with no contraception due to the dramatically decreased rate of pregnancy overall. However, when pregnancy does occur with a copper IUD in place, a higher percentage are ectopic, approximately 3-4%. If a pregnancy continues with the IUD in place, there is an increased risk of complications including preterm delivery, chorioamnionitis, and spontaneous abortion. If the IUD is removed, these risks are lower.[5]
Contraindications
[edit]The copper IUD is considered safe and effective in lactation and in those who have never been pregnant. In the World Health Organization (WHO) Medical Eligibility Criteria for Contraceptive Use category 3 contraindications (risk typically outweighs benefit) and category 4 contraindications (unacceptable health risk) are listed for the copper IUD. Category 3 contraindications include untreated HIV/AIDS, recent and recurrent exposure to gonorrhea or chlamydia without adequate treatment, benign gestational trophoblastic disease, and ovarian cancer. Category 4 contraindications besides pregnancy and active genital tract infections (e.g. pelvic tuberculosis, STIs, endometritis) include malignant gestational trophoblastic disease, abnormal uterine bleeding, active cervical cancer, Wilson's disease, and active endometrial cancer. HIV infection is not itself a contraindication, as long as it is treated. There are not known drug interactions between the copper IUD and anti-retroviral medications.[18][30]
Device description
[edit]There are a number of models of copper IUDs available around the world. Most copper devices consist of a plastic (polyethylene) core that is wrapped in a copper wire.[12] Many of the devices have a T-shape similar to the hormonal IUD. However, there are "frameless" copper IUDs available as well. ParaGard is the only model currently available in the United States. At least three copper IUD models are available in Canada, two of which are slimmer T-shape versions used for those who have not had children. Early copper IUDs had copper around only the vertical stem, but more recent models have copper sleeves wrapped around the horizontal arms as well, increasing surface area and thereby effectiveness.[31]
Insertion
[edit]A copper IUD can be inserted at any phase of the menstrual cycle, as long as pregnancy can be reliably excluded. It may be inserted in the immediate postpartum period (shortly after delivery of the placenta), and after an induced or spontaneous abortion provided a genital tract infection can be reliably excluded.[5] NSAIDs taken prior to the procedure and use of local anesthesia are recommended to reduce pain at the time of insertion.[14][32][27]
Types
[edit]
Many different types of copper IUDs are currently manufactured worldwide, but availability varies by country. In the United States, only one type of copper IUD is approved for use, while in the United Kingdom, over ten varieties are available.[33] One company, Mona Lisa N.V., offers generic versions of many existing IUDs.[34] Additionally, SMB Corporation of India[35] manufactures copper IUDs for markets in India and other regions.
| IUD | Type | Width
(mm) |
Length (mm) | Copper (mm2) | Life (years) | Manufacturer | Distinguishing characteristics |
|---|---|---|---|---|---|---|---|
| Gyneplus Cu 380 | T-shaped | 380 | 5 | Dispo.Cont. | |||
| Multiload Cu375 | U-shaped | 20.5[36] | 35 | 375 | 5 | Multilan | |
| Multiload Cu250 | U-shaped | 250 | 3 | Multilan | |||
| Multi-Safe 375 | U-shaped | 19.5[37] | 32.5 | 375 | 5 | Mona Lisa N.V. | |
| Multi-Safe 375 Short Loop | U-shaped | 19,5 | 29,4 | 375 | 5 | Mona Lisa N.V. | |
| Load 375 | U-shaped | 19.5[37] | 32.5 | 375 | 5 | 7-MED Industrie | |
| Nova-T 380 | T-shaped (plain) | 32[38] | 32 | 380 | 5 | Bayer | |
| Neo-Safe T 380 | T-shaped (plain) | 32[37] | 32 | 380 | 5 | Mona Lisa N.V. | |
| Neo-Safe T 380 Mini | T-shaped (plain) | 24[39] | 30 | 380 | 5 | Mona Lisa N.V. | |
| UT 380 | T-shaped (plain) | 32[37] | 32 | 380 | 5 | Laboratoire CCD | |
| UT 380 Short | T-shaped (plain) | 32[37] | 28.4 | 380 | 5 | Laboratoire CCD | |
| Flexi-T 300 | T-shaped (plain) | 23[40] | 29 | 300 | 5 | Prosan | |
| Flexi-T + 300 | T-shaped (plain) | 28[40] | 32 | 300 | 5 | Prosan | Wider arms than Flexi-T 300 |
| T-safe CU 380A | T-shaped (banded) | 31.8[37] | 35.8 | 380 | 10 | Mona Lisa N.V. | |
| T-safe CU 380A QL | T-shaped (banded) | 31.8[37] | 35.8 | 380 | 10 | Mona Lisa N.V. | |
| Flexi-T + 380 | T-shaped (banded) | 28[40] | 32 | 380 | 5 | Prosan | |
| TT 380 Slimline | T-shaped (banded) | 31.8[37] | 35.8 | 380 | 10 | 7-MED Industrie | |
| TT 380 Mini | T-shaped (banded) | 23.2[37] | 29.5 | 380 | 5 | 7-MED Industrie | |
| Paragard | T-shaped (banded) | 32[41] | 36 | 380 | 10 | Duramed | Only copper IUD approved by the US FDA[citation needed] |
| Gynefix 330 | Frameless | 2.2[37] | 30 | 330 | 5 | Contrel | Only frameless IUD brand available[citation needed] |
| Gynefix 200 | Frameless | 200 | 5 | Contrel | Only frameless IUD brand available[citation needed] | ||
| IUB SCu300A/B | Spherical (3D) | 300 | 5 | OCON | Nitinol alloy cored frame. Brand name is Ballerine. | ||
| SMB TCu 380A | T-shaped (banded) | 32[42] | 36 | 380 | 10 | SMB corp | WHO UNFPA Prequalified IUD Manufacturer |
| Protect TCu 380A | T-shaped (banded) | 380 | 12 | SMB corp | WHO UNFPA Prequalified IUD Manufacturer | ||
| Protect Multi-arm Cu 375 standard | U-shaped | 375 | 5 | SMB corp | WHO UNFPA Prequalified IUD Manufacturer | ||
| Protect Multi-arm Cu 375 short | U-shaped | 375 | 5 | SMB corp | WHO UNFPA Prequalified IUD Manufacturer |
Frameless IUDs
[edit]The frameless IUD eliminates the use of the frame that gives conventional IUDs their signature T-shape. This change in design was made to reduce discomfort and expulsion associated with prior IUDs; without a solid frame, the frameless IUD should mold to the shape of the uterus. It may reduce expulsion and discontinuation rates compared to framed copper IUDs.[43]
Gynefix is the only frameless IUD brand currently available. It consists of hollow copper tubes on a polypropylene thread. It is inserted through the cervix with a special applicator that anchors the thread to the fundus (top) of the uterus; the thread is then cut with a tail hanging outside of the cervix, similar to framed IUDs, or looped back into the cervical canal for patient comfort. When this tail is pulled, the anchor is released and the device can be removed. This requires more force than removing a T-shaped IUD and results in comparable discomfort during removal.[44]
Mechanism of action
[edit]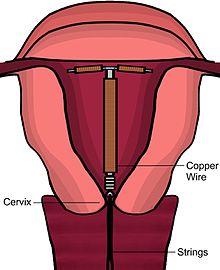
The copper IUD's primary mechanism of action is to prevent fertilization.[5][22][45][46][47] Copper causes a localized inflammatory response, which is spermicidal and causes the endometrium to be inhospitable.[5][22][18][45]
Although not a primary mechanism of action, copper may disrupt embryonic implantation,[5][48] especially when used for emergency contraception.[49][50] However, if implantation occurs, there is no evidence that copper affects subsequent development of a pregnancy or causes embryonic failure.[5][45] Therefore, the copper IUD is considered to be a true contraceptive and not an abortifacient.[5][22]
Usage
[edit]Globally, the IUD is the most widely used method of reversible birth control.[51] The most recent data indicates that there are 169 million IUD users around the world. This includes both the nonhormonal and hormonal IUDs. IUDs are most popular in Asia, where the prevalence is almost 30%. In Africa and Europe, the prevalence is around 20%.[51] As of 2009, levels of IUD use in the United States are estimated to be 5.5%.[15] Data in the United States does not distinguish between hormonal and non-hormonal IUDs. In Europe, copper IUD prevalence ranges from under 5% in the United Kingdom to over 10% in Denmark in 2006.[52]
History
[edit]According to popular legend, Arab traders inserted small stones into the uteruses of their camels to prevent pregnancy during long desert treks. The story was originally a tall tale to entertain delegates at a scientific conference on family planning; although it was later repeated as truth, it has no known historical basis.[53]
Precursors to IUDs were first marketed in 1902. Developed from stem pessaries (where the stem held the pessary in place over the cervix), the 'stem' on these devices actually extended into the uterus itself. Because they occupied both the vagina and the uterus, this type of stem pessary was also known as an intrauterine device. The use of intrauterine devices was associated with high rates of infection; for this reason, they were condemned by the medical community.[54]
The first intrauterine device (contained entirely in the uterus) was described in a German publication in 1909, although the author appears to have never marketed his product.[55]
In 1929, Ernst Gräfenberg of Germany published a report on an IUD made of silk sutures. He had found a 3% pregnancy rate among 1,100 women using his ring. In 1930, Gräfenberg reported a lower pregnancy rate of 1.6% among 600 women using an improved ring wrapped in silver wire. Unbeknownst to Gräfenberg, the silver wire was contaminated with 26% copper. Copper's role in increasing IUD efficacy would not be recognized until nearly 40 years later.
In 1934, Japanese physician Tenrei Ota developed a variation of Gräfenberg's ring that contained a supportive structure in the center. The addition of this central disc lowered the IUD's expulsion rate. These devices still had high rates of infection, and their use and development were further stifled by World War II politics: contraception was forbidden in both Nazi Germany and Axis-allied Japan. The Allies did not learn of the work by Gräfenberg and Ota until well after the war ended.[55]
The first plastic IUD, the Margulies Coil or Margulies Spiral, was introduced in 1958. This device was somewhat large, causing discomfort to a large proportion of women users, and had a hard plastic tail, causing discomfort to their male partners. The modern colloquialism "coil" is based on the coil-shaped design of early IUDs.
The Lippes Loop, a slightly smaller device with a monofilament tail, was introduced in 1962 and gained in popularity over the Margulies device.[54]
The stainless steel single-ring IUD was developed in the 1970s[56] and widely used in China because of low manufacturing costs. The Chinese government banned production of steel IUDs in 1993 due to high failure rates (up to 10% per year).[14][57]
Howard Tatum, in the US, conceived the plastic T-shaped IUD in 1968. Shortly thereafter Jaime Zipper, in Chile, introduced the idea of adding copper to the devices to improve their contraceptive effectiveness.[54][58] It was found that copper-containing devices could be made in smaller sizes without compromising effectiveness, resulting in fewer side effects such as pain and bleeding.[14] T-shaped devices had lower rates of expulsion due to their greater similarity to the shape of the uterus.[55]
The poorly designed Dalkon Shield plastic IUD (which had a multifilament tail) was manufactured by the A. H. Robins Company and sold by Robins in the United States for three and a half years from January 1971 through June 1974, before sales were suspended by Robins on June 28, 1974, at the request of the FDA because of safety concerns following reports of 110 septic spontaneous abortions in women with the Dalkon Shield in place, seven of whom had died.[59][60] Robins stopped international sales of the Dalkon Shield in April 1975.[61]
Tatum developed many different models of the copper IUD. He created the TCu220 C, which had copper collars as opposed to a copper filament, which prevented metal loss and increased the lifespan of the device. Second-generation copper-T IUDs were also introduced in the 1970s. These devices had higher surface areas of copper, and for the first time consistently achieved effectiveness rates of greater than 99%.[14] The last model Tatum developed was the TCu380A, the model that is most recommended today.[12]
Brands
[edit]This section needs additional citations for verification. (July 2023) |
The Paragard T-380A is an IUD with copper, manufactured and marketed in the United States by The Cooper Companies.[62] It is the only copper-containing intrauterine device approved for use in the U.S. (four hormonal uterine devices, Mirena, Skyla, Liletta, and Kyleena are also approved).[63] The Paragard consists of a T-shaped polyethylene frame wound with copper wire, along with two monofilament threads to aid in the removal of the IUD.
The Paragard T 380A was developed in the 1970s by the Population Council and Finishing Enterprises Inc. (FEI).[64] The Population Council's Paragard new drug application (NDA) was approved by the U.S. Food and Drug Administration (FDA) and FEI began manufacturing it for distribution outside the United States in 1984.[65] GynoPharma (originally GynoMed) began marketing it in the U.S. in May 1988. On August 2, 1995, Ortho-McNeil acquired GynoPharma and began marketing Paragard in the U.S.[66] On January 1, 2004, FEI Women's Health acquired the patent from the Population Council and U.S. marketing rights from Ortho-McNeil.[67] On November 10, 2005, Duramed Pharmaceuticals, a subsidiary of Barr Pharmaceuticals, acquired FEI Women's Health and Paragard.[68] On July 18, 2008, it was announced that Teva Pharmaceutical Industries Ltd. would acquire Barr Pharmaceuticals.[69]
On November 1, 2017, The Cooper Companies acquired Paragard from Teva Pharmaceutical Industries for approximately $1.1 billion.[70]
The original FDA approval of Paragard in 1984 was for 4 years continuous use, this was later extended to 6 years in 1989, then 8 years in 1991, then 10 years in 1994. (ATC code G02BA02 (WHO))
References
[edit]- ^ a b c d e f g h Goodwin TM, Montoro MN, Muderspach L, Paulson R, Roy S (2010). Management of Common Problems in Obstetrics and Gynecology (5 ed.). John Wiley & Sons. pp. 494–496. ISBN 978-1-4443-9034-6. Archived from the original on 2017-11-05.
- ^ a b Trussell J (2011). "Contraceptive efficacy" (PDF). In Hatcher RA, Trussell J, Nelson AL, Cates W Jr, Kowal D, Policar MS (eds.). Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 779–863. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734. Archived (PDF) from the original on 2017-02-15.
- ^ a b c d e World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 370–2. hdl:10665/44053. ISBN 9789241547659.
- ^ a b Wipf J (2015). Women's Health, An Issue of Medical Clinics of North America. Elsevier Health Sciences. p. 507. ISBN 978-0-323-37608-2. Archived from the original on 2017-09-24.
- ^ a b c d e f g h i j k l m n "Long-Acting Reversible Contraception Implants and Intrauterine Devices: Practice Bulletin #186". American College of Obstetricians and Gynecologists. 2024. Retrieved 2025-01-28.
- ^ "IUD Birth Control Info About Mirena & ParaGard IUDs". www.plannedparenthood.org. Archived from the original on 4 January 2021. Retrieved 22 March 2018.
- ^ a b c British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 557–559. ISBN 978-0-85711-156-2.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Speroff L, Darney PD (2011). A Clinical Guide for Contraception. Lippincott Williams & Wilkins. p. 243. ISBN 978-1-60831-610-6. Archived from the original on 2017-11-05.
- ^ Schäfer-Korting M (2010). Drug Delivery. Springer Science & Business Media. p. 290. ISBN 978-3-642-00477-3. Archived from the original on 2017-11-05.
- ^ Winner, B, Peipert, JF, Zhao, Q, Buckel, C, Madden, T, Allsworth, JE, Secura, GM. (2012). "Effectiveness of Long-Acting Reversible Contraception". New England Journal of Medicine. 366 (21): 1998–2007. doi:10.1056/NEJMoa1110855. PMID 22621627. S2CID 16812353. Archived from the original on 2020-08-17. Retrieved 2019-08-18.
- ^ a b c d e Kulier R, O'Brien P, Helmerhorst FM, Usher-Patel M, d'Arcangues C (2008). "Copper containing, framed intra-uterine devices for contraception (Review)". Cochrane Database of Systematic Reviews (4): CD005347. doi:10.1002/14651858.CD005347.PUB3. PMID 17943851.
- ^ O'Brien PA, Marfleet C (25 January 2005). "Frameless versus classical intrauterine device for contraception". The Cochrane Database of Systematic Reviews (1): CD003282. doi:10.1002/14651858.CD003282.pub2. ISSN 1469-493X. PMID 15674904.
- ^ a b c d e Treiman K, Liskin L, Kols A, Rinehart W (December 1995). "IUDs—an update" (PDF). Population Reports. Series B, Intrauterine Devices (6). Baltimore: Johns Hopkins School of Public Health, Population Information Program: 1–35. PMID 8724322. Archived (PDF) from the original on October 29, 2013. Retrieved July 9, 2006.
- ^ a b The Guttmacher Institute (2012). "Contraceptive Use in the United States". Archived from the original on 2013-10-04. Retrieved 2013-10-04.
- ^ Bartz D, Greenberg JA (2008). "Sterilization in the United States". Reviews in Obstetrics & Gynecology. 1 (1): 23–32. PMC 2492586. PMID 18701927.
- ^ Committee On Practice Bulletins-Gynecology LA (November 2017). "Practice Bulletin No. 186" (PDF). Obstetrics & Gynecology. 130 (5): e251 – e269. doi:10.1097/AOG.0000000000002400. PMID 29064972. S2CID 35477591. Archived from the original on 2021-08-28. Retrieved 2019-06-20.
- ^ a b c d e Hoffman BL, Schorge JO, Schaffer JI, Halvorson LM, Bradshaw KD, Cunningham FG, Calver LE, eds. (2012). Williams Gynecology (2nd ed.). McGraw-Hill Medical. ISBN 978-0-07-171672-7.
- ^ Lippes, J, Malik, T, Tatum, HJ (1976). "The postcoital copper-T". Adv Plan Parent. 11 (1): 24–9. PMID 976578.
- ^ Cheng, L, Gulmezoglu, AM, Piaggio, G, Ezcurra, E, Van Look, PF (2008). Cheng L (ed.). "Interventions for emergency contraception". Cochrane Database of Systematic Reviews (2): CD001324. doi:10.1002/14651858.cd001324.pub3. PMID 18425871.
- ^ a b Cleland K, Zhu H, Goldstruck N, Cheng L, Trussel T (2012). "The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience". Human Reproduction. 27 (7): 1994–2000. doi:10.1093/humrep/des140. PMC 3619968. PMID 22570193.
- ^ a b c d e f g h Dean G, Schwarz EB (2011). "Intrauterine contraceptives (IUCs)". In Hatcher RA, Trussell J, Nelson AL, Cates W Jr, Kowal D, Policar MS (eds.). Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 147–191. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734. p.150:
Mechanism of action
Although the precise mechanism of action is not known, currently available IUCs work primarily by preventing sperm from fertilizing ova.26 IUCs are not abortifacients: they do not interrupt an implanted pregnancy.27 Pregnancy is prevented by a combination of the "foreign body effect" of the plastic or metal frame and the specific action of the medication (copper or levonorgestrel) that is released. Exposure to a foreign body causes a sterile inflammatory reaction in the intrauterine environment that is toxic to sperm and ova and impairs implantation.28,29 The production of cytotoxic peptides and activation of enzymes lead to inhibition of sperm motility, reduced sperm capacitation and survival, and increased phagocytosis of sperm.30,31 The TCu380A causes an increase in copper ions, enzymes, prostaglandins, and white blood cells (macrophages) in uterine and tubal fluids; these impair sperm function and prevent fertilization.
p. 162:
Table 7-1. Myths and misconceptions about IUCs
Myth: IUCs are abortifacients. Fact: IUCs prevent fertilization and are true contraceptives. - ^ Belhadj, H, et al. (1986). "Recovery of fertility after use of the Levonorgestrel 20 mcg/d or copper T 380 Ag intrauterine device". Contraception. 34 (3): 261–267. doi:10.1016/0010-7824(86)90007-7. PMID 3098498.
- ^ Kaneshiro B, Aeby T (2010). "Long-term safety, efficacy, and patient acceptability of the intrauterine Copper T-380A contraceptive device". International Journal of Women's Health. 2: 211–220. doi:10.2147/ijwh.s6914. PMC 2971735. PMID 21072313.
- ^ Bahamondes L, Díaz J, Marchi NM, Petta CA, Cristofoletti ML, Gomez G (November 1995). "Performance of copper intrauterine devices when inserted after an expulsion". Hum. Reprod. 10 (11): 2917–8. doi:10.1093/oxfordjournals.humrep.a135819. PMID 8747044.
- ^ Berger-Kulemann V, Einspieler H, Hachemian N, Prayer D, Trattnig S, Weber M, Ba-Ssalamah A (2013). "Magnetic Field Interactions of Copper-Containing Intrauterine Devices in 3.0-Tesla Magnetic Resonance Imaging: In Vivo Study". Korean Journal of Radiology. 14 (3): 416–22. doi:10.3348/kjr.2013.14.3.416. ISSN 1229-6929. PMC 3655294. PMID 23690707.
- ^ a b Mohllajee AP, Curtis KM, Peterson HB (2006). "Does insertion of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review". Contraception. 73 (2): 143–153. doi:10.1016/j.contraception.2005.08.007. PMID 16413845. Archived from the original on 2020-02-06. Retrieved 2020-09-30.
- ^ "Infection Prevention Practices for IUD Insertion and Removal". Archived from the original on 2010-01-01. By the United States Agency for International Development (USAID). Retrieved on Feb 14, 2010
- ^ Christelle K, Norhayati MN, Jaafar SH (2022-08-26). Cochrane Fertility Regulation Group (ed.). "Interventions to prevent or treat heavy menstrual bleeding or pain associated with intrauterine-device use". Cochrane Database of Systematic Reviews. 2022 (8): CD006034. doi:10.1002/14651858.CD006034.pub3. PMC 9413853. PMID 36017945.
- ^ World Health Organization (2015). Medical eligibility criteria for contraceptive use (5th ed.). Geneva: World Health Organization. hdl:10665/181468. ISBN 9789241549158.
- ^ Sivin I, Stern J (1979). "Long-acting, more effective Copper T IUDs: a summary of U.S. experience, 1970–1975". Studies in Family Planning. 10 (10): 263–281. doi:10.2307/1965507. JSTOR 1965507. PMID 516121.
- ^ Hutten-Czapski P, Goertzen J (2008). "The occasional intrauterine contraceptive device insertion" (PDF). Can J Rural Med. 13 (1): 31–5. PMID 18208650. Archived from the original (PDF) on 2016-08-14. Retrieved 2009-01-22.
- ^ "Contraceptive coils (IUDs)". NetDoctor.co.uk. 2006. Archived from the original on 2006-07-17. Retrieved 2006-07-05.
- ^ Marketing e (2024-10-25). "Mona Lisa N.V." Website Mona Lisa. Retrieved 2024-10-25., a manufacturer of generic IUDs
- ^ "SMB T 380A, Copper T IUD, SMB T 380A IUD, Copper T IUD Device". www.smbcorpn.com. Archived from the original on 2020-07-22. Retrieved 2020-07-21.
- ^ "Data" (PDF). www.broadwaymed.co.nz. Archived from the original (PDF) on 2020-01-17. Retrieved 2020-07-10.
- ^ a b c d e f g h i j "Guidance" (PDF). www.nhstaysideadtc.scot.nhs.uk. Archived (PDF) from the original on 2021-01-10. Retrieved 2020-07-10.
- ^ "Nova-T 380 IUD (Intrauterine Device)". www.mistrymedical.com. Archived from the original on 2020-03-30. Retrieved 2020-03-30.
- ^ "Neo-Safe T CU 380 Mini IUD". MidMeds Ltd. Archived from the original on 2020-03-30. Retrieved 2020-03-30.
- ^ a b c "Product information". Prosan (in Dutch). Archived from the original on 2020-03-30. Retrieved 2020-03-29.
- ^ "How big is Paragard?". Paragard IUD. Archived from the original on 2020-03-30. Retrieved 2020-03-29.
- ^ "SMB T 380A, Copper T IUD, SMB T 380A IUD, Copper T IUD Device". www.smbcorpn.com. Archived from the original on 2020-07-22. Retrieved 2020-07-21.
- ^ Wu S, Hu J, Wildemeersch D (February 2000). "Performance of the frameless GyneFix and the TCu380A IUDs in a 3-year multicenter, randomized, comparative trial in parous women". Contraception. 61 (2): 91–8. doi:10.1016/s0010-7824(00)00087-1. PMID 10802273.
- ^ D'Souza RE, Bounds W, Guillebaud J (April 2003). "Comparative trial of the force required for, and pain of, removing GyneFix versus Gyne-T380S following randomised insertion". J Fam Plann Reprod Health Care. 29 (2): 29–31. doi:10.1783/147118903101197494. PMID 12681034.
- ^ a b c Ortiz ME, Croxatto HB (June 2007). "Copper-T intrauterine device and levonorgestrel intrauterine system: biological bases of their mechanism of action". Contraception. 75 (6 Suppl): S16‒S30. doi:10.1016/j.contraception.2007.01.020. PMID 17531610. p. S28:
Conclusions
Active substances released from the IUD or IUS, together with products derived from the inflammatory reaction present in the luminal fluids of the genital tract, are toxic for spermatozoa and oocytes, preventing the encounter of healthy gametes and the formation of viable embryos. The current data do not indicate that embryos are formed in IUD users at a rate comparable to that of nonusers. The common belief that the usual mechanism of action of IUDs in women is the destruction of embryos in the uterus is not supported by empirical evidence. The bulk of the data indicate that interference with the reproductive process after fertilization has taken place is exceptional in the presence of a T-Cu or LNG-IUD and that the usual mechanism by which they prevent pregnancy in women is by preventing fertilization. - ^ Speroff L, Darney PD (2011). "Intrauterine contraception". A clinical guide for contraception (5th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 239–280. ISBN 978-1-60831-610-6. p. 246:
Mechanism of action
The contraceptive action of all IUDs is mainly in the intrauterine cavity. Ovulation is not affected, and the IUD is not an abortifacient.58–60 It is currently believed that the mechanism of action for IUDs is the production of an intrauterine environment that is spermicidal.
Nonmedicated IUDs depend for contraception on the general reaction of the uterus to a foreign body. It is believed that this reaction, a sterile inflammatory response, produces tissue injury of a minor degree but sufficient to be spermicidal. Very few, if any, sperm reach the ovum in the fallopian tube… In women using copper IUDs, sensitive assays for human chorionic gonadotropin do not find evidence of fertilization.62,63 This is consistent with the fact that the copper IUD protects against both intrauterine and ectopic pregnancies.
The copper IUD releases free copper and copper salts that have both a biochemical and morphological impact on the endometrium and also produce alterations in cervical mucus and endometrial secretions... An additional spermicidal effect probably takes place in the cervical mucus. - ^ Jensen JT, Mishell DR Jr (2012). "Family planning: contraception, sterilization, and pregnancy termination". In Lentz GM, Lobo RA, Gershenson DM, Katz VL (eds.). Comprehensive gynecology. Philadelphia: Mosby Elsevier. pp. 215–272. ISBN 978-0-323-06986-1. p. 259:
Intrauterine devices
Mechanisms of action
The common belief that the usual mechanism of action of IUDs in women is destruction of embryos in the uterus is not supported by empirical evidence... Because concern over the mechanism of action represents a barrier to acceptance of this important and highly effective method for some women and some clinicians, it is important to point out that there is no evidence to suggest that the mechanism of action of IUDs is abortifacient... the principal mechanism of action of the copper T 380A IUD is to interfere with sperm action, preventing fertilization of the ovum. - ^ ESHRE Capri Workshop Group (May–June 2008). "Intrauterine devices and intrauterine systems". Human Reproduction Update. 14 (3): 197‒208. doi:10.1093/humupd/dmn003. PMID 18400840. p. 199:
Mechanisms of action
Thus, both clinical and experimental evidence suggests that IUDs can prevent and disrupt implantation. It is unlikely, however, that this is the main IUD mode of action, … The best evidence indicates that in IUD users it is unusual for embryos to reach the uterus.
In conclusion, IUDs may exert their contraceptive action at different levels. Potentially, they interfere with sperm function and transport within the uterus and tubes. It is difficult to determine whether fertilization of the oocyte is impaired by these compromised sperm. There is sufficient evidence to suggest that IUDs can prevent and disrupt implantation. The extent to which this interference contributes to its contraceptive action is unknown. The data are scanty and the political consequences of resolving this issue interfere with comprehensive research.
p. 205:
Summary
IUDs that release copper or levonorgestrel are extremely effective contraceptives... Both copper IUDs and levonorgestrel-releasing IUSs may interfere with implantation, although this may not be the primary mechanism of action. The devices also create barriers to sperm transport and fertilization, and sensitive assays detect hCG in less than 1% of cycles, indicating that significant prevention must occur before the stage of implantation. - ^ Speroff L, Darney PD (2011). "Special uses of oral contraception: emergency contraception, the progestin-only minipill". A clinical guide for contraception (5th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 153–166. ISBN 978-1-60831-610-6. p. 157:
Emergency postcoital contraception
Other methods
Another method of emergency contraception is the insertion of a copper IUD, anytime during the preovulatory phase of the menstrual cycle and up to 5 days after ovulation. The failure rate (in a small number of studies) is very low, 0.1%.34,35 This method definitely prevents implantation, but it is not suitable for women who are not candidates for intrauterine contraception, e.g., multiple sexual partners or a rape victim. The use of a copper IUD for emergency contraception is expensive, but not if it is retained as an ongoing method of contraception. - ^ Trussell J, Schwarz EB (2011). "Emergency contraception". In Hatcher RA, Trussell J, Nelson AL, Cates W Jr, Kowal D, Policar MS (eds.). Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 113–145. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734. p. 121:
Mechanism of action
Copper-releasing IUCs
When used as a regular or emergency method of contraception, copper-releasing IUCs act primarily to prevent fertilization. Emergency insertion of a copper IUC is significantly more effective than the use of ECPs, reducing the risk of pregnancy following unprotected intercourse by more than 99%.2,3 This very high level of effectiveness implies that emergency insertion of a copper IUC must prevent some pregnancies after fertilization.
Pregnancy begins with implantation according to medical authorities such as the US FDA, the National Institutes of Health79 and the American College of Obstetricians and Gynecologists (ACOG).80 - ^ a b Brigid Fitzgerald Reading. "Growth in World Contraceptive Use Stalling; 215 Million Women's Needs Still Unmet". Earth Policy Institute. Archived from the original on 2012-12-03. Retrieved 2013-10-04.
- ^ Sonfield, Adam (2012). "Popularity Disparity: Attitudes About the IUD in Europe and the United States". The Guttmacher Institute. Archived from the original on 2010-03-07.
- ^ "A History of Birth Control Methods". Planned Parenthood. June 2002. Archived from the original on 2008-05-17. Retrieved 2007-10-14., which cites:
- Thomas P (1988-03-14). "Contraceptives". Medical World News. 29 (5): 48.
- Bullough VL, Bullough, Bonnie (1990). Contraception: A Guide to Birth Control Methods. Buffalo, NY: Prometheus Books. ISBN 9780879755898.
- ^ a b c Lynch CM. "History of the IUD". Contraception Online. Baylor College of Medicine. Archived from the original on 2006-01-27. Retrieved 2006-07-09.
- ^ a b c "Evolution and Revolution: The Past, Present, and Future of Contraception". Contraception Online (Baylor College of Medicine). 10 (6). February 2000. Archived from the original on September 26, 2006.
- ^ Bradley J (August 1998). "Ultrasound Interactive Case Study: Ring IUD". OBGYN.net. Archived from the original on 2006-01-17. Retrieved 2006-07-09. (Has pictures of various IUD designs.)
- ^ Kaufman J (May–Jun 1993). "The cost of IUD failure in China". Studies in Family Planning. 24 (3): 194–6. doi:10.2307/2939234. JSTOR 2939234. PMID 8351700.
- ^ Van Kets H (1997). C. Coll Capdevila, L. Iglesias Cortit, G. Creatsas (eds.). "Importance of intrauterine contraception". Contraception Today, Proceedings of the 4th Congress of the European Society of Contraception. The Parthenon Publishing Group. pp. 112–116. Archived from the original on 2006-08-10. Retrieved 2006-07-09. (Has pictures of many IUD designs, both historic and modern.)
- ^ Sivin I (1993). "Another look at the Dalkon Shield: meta-analysis underscores its problems". Contraception. 48 (1): 1–12. doi:10.1016/0010-7824(93)90060-K. PMID 8403900.
- ^ Mintz, Morton (January 15, 1986). "A Crime Against Women. A. H. Robins and the Dalkon Shield". Multinational Monitor. 7 (1). Archived from the original on October 3, 2006.
- ^ Salem R (February 2006). "New Attention to the IUD: Expanding Women's Contraceptive Options To Meet Their Needs". Popul Rep B (7). Archived from the original on 2007-10-13.
- ^ "Paragard® (intrauterine copper contraceptive) - CooperSurgical". www.coopersurgical.com. 2023-06-28. Retrieved 2023-11-29.
- ^ "IUD Birth Control | Info About Mirena & Paragard IUDs". www.plannedparenthood.org. Retrieved 2023-11-29.
- ^ "The TCu380A Intrauterine Contraceptive Device (IUD): Specification, Prequalification and Guidelines for Procurement, 2010" (PDF).
- ^ Center for Drug Evaluation and Research. "Approval Package for Application Number 18-680" (PDF).
- ^ "Health: Birth Control; U.S. Experts Applaud Growth in Options for Contraception". The New York Times.
- ^ "20f". www.sec.gov. 2005-06-29. Retrieved 2023-11-29.
- ^ "Duramed Pharmaceuticals, Inc. Completes Acquisition Of FEI Company Women's Health And ParaGard(R) IUD Product". BioSpace. Retrieved 2023-11-29.
- ^ Processing P (2008-12-23). "Teva Completes Acquisition of Barr". Pharmaceutical Processing World. Retrieved 2023-11-29.
- ^ "The Cooper Companies Completes Acquisition of Paragard IUD From Teva". Cooper Companies. Archived from the original on 2021-11-24. Retrieved 2021-11-24.
