Wilfred Stein
Wilfred Donald Stein | |
|---|---|
 Stein in 2015 | |
| Born | Wilfred Donald Stein November 26, 1931 |
| Alma mater | |
| Notable work |
|
| Spouse |
Chana Morgenstern (m. 1954) |
| Awards |
|
| Era | 21st-century philosophy |
| Region | Biophysics |
| School | |
| Institutions | Hebrew University |
Main interests |
|
Notable ideas | cation occlusion, osmotic engines, the cell membrane as a fluid, amphiphilic structure |
Wilfred D. Stein is a writer[1] and biophysicist who has applied mathematical principles to medical, biologic, and oncologic problems.[2]
Early life and education
[edit]Wilfred Donald Stein was born on 26 November 1931 in Durban, South Africa to Philip, a mathematician, and Lily (née Rolnick) Stein. His parents emigrated from Lithuania as young children. He was the third of his siblings, Sylvester Stein (born 25 December 1920) and Zena Stein (born 7 July 1922). Wilfred Stein attended the University of the Witwatersrand (Wits), Johannesburg SA, receiving his MSc degree in 1954 based on his thesis "Melanogenesis and the structure of the melanin granue." [3] In 1954, he married Chana Morgenstern, born 27 October 1934, before leaving South Africa to study in London, UK. They had four children, Aryeh David (b. 1956), Moshe Baruch (b 1960), Gideon Paschal (b. 1962) and Rebecca Miriam (b. 1966). He has 9 grandchildren. [4] [5]
Scientific career
[edit]Stein received his M.Sc. degree in 1954 in Physiological Chemistry from the University of the Witwatersrand, and his PhD degree from King's College London in 1958 in Biophysics. He did postdoctoral work in University of Cambridge UK and University of Michigan Ann Arbor, before taking up a position as Assistant Professor at the University of Manchester UK (1963–1968). He moved to Israel in late 1968, joining the faculty of the Alexander Silberman Institute of Life sciences, Hebrew University of Jerusalem in 1969, where he remained until his retirement in 2006. He taught biochemistry, biophysics and physiology. He also taught at the Weizmann Institute in Israel, where he continued as a consultant through 2003.[6]
Following his retirement, Stein has continued to bring his mathematical perspective to medical problems, both while working as a Professor Emeritus at Hebrew University and including also a number of Visiting Professorships and Sabbatical fellowships in the laboratories of Dr. Michael Lanzer, Dr. Igor Roninson, Dr. Michael Gottesman, Dr. Thomas Zeuthen, Dr. Susan Bates and Dr. Tito Fojo.[7] [8]
Besides Stein's contribution to concepts in kinetics, biology, and medicine, he also, together with Eric Barnard, conducted work on the first labeling of the active center of an enzyme, ribonuclease, identifying histidine residue 119.[9] He is the author of more than 300 peer-reviewed publications, 9 books on topics in science, and 2 family memoirs.
Research accomplishments
[edit]Membrane transport physiology
[edit]Conceptualization and mathematical modeling of how membrane channels, carriers and pumps work to transport molecules across the cell membrane. Stein was the first to propose a model of the cell membrane as a fluid, amphiphilic structure. He presented this idea at the Society of General Physiology meeting in 1968, where the chairman of the session said: "Let’s stop here and discuss this interesting new idea" .[10] Stein's model, however, was not as cogent as the fluid mosaic model some years later presented by Singer and Nicolson, who are credited with the idea in text-books.[11][12]
The kinetic equations of membrane transport were developed by Stein, together with William Lieb, and published in “Transport and Diffusion Across Cell Membranes”, a comprehensive treatment of transport kinetics.[13]
Na,K-pump (Na,K-ATPase)
[edit]In collaboration with Steven Karlish at the Weizmann Institute, Stein investigated the kinetic mechanism of active Na and K ion transport, confirming the basic alternating access model of active Na and K transport. [14] [15] An important finding was that trapping or “occlusion” of the K and Na ions in the protein has the functional role of minimizing wasteful cation leakages through the system, thus ensuring optimal efficiency of energy coupling between ATP (Adenosine Triphosphate) hydrolysis and active Na and K ion movements, 3 Na ions out of and 2 K ions into the cell per cycle, respectively. [16] Cation occlusion is now considered an essential property of all ATP-driven cation pumps and also other coupled cation transport systems (e.g. cation exchangers or co-transport proteins).
Flux of calcium across calcium-transporting cells
[edit]Stein collaborated with Felix Bronner, an authority on the physiology of calcium movements, in developing a model that could account for the rates of transmembrane movements of calcium in the face of the cell's extremely low concentrations of the ion. They postulated that the role of the cell's calcium-binding proteins was to raise effective calcium concentrations and thus provide the necessary high transcellular calcium fluxes.[17]
Coupling of salt/substrate and water transport in membrane proteins
[edit]As a visiting scientist at the University of Copenhagen in 1993, Stein worked with the Danish physiologist Thomas Zeuthen on the coupling of water and substrates in membrane proteins.[18] They provided a conceptual framework for how a flux of substrates through a membrane protein can lead to a co-flux of water. Zeuthen & Stein suggested that the substrate-flux generates a hyperosmolar compartment within the protein, i.e. in the aqueous cavities abutting the outer solutions. As a result, water enters this compartment by osmosis and proceeds across the membrane.
ABC transporter kinetics
[edit]In collaboration with Thomas Litman, at the University of Copenhagen, Stein worked out the kinetics of the ABC transporter P-glycoprotein (ABCB1) based on transport and ATPase measurements. [19]
The two often observed co-operative behavior between 2 substrates, and found that kinetics included non-competitive, competitive, and allosteric interactions. [20]
A two-step model for pumping of drugs by P-glycoprotein
[edit]In a collaboration with Suresh Ambudkar and his group, Stein presented estimates of the turnover numbers for ATP hydrolysis and drug transport by P-glycoprotein, leading them to conclude that more than a single ATP molecule was hydrolyzed for each drug molecule pumped.[21] In further collaboration with Litman, and based on detailed kinetic measurements of drug accumulation in cell lines with different levels of the multidrug resistance transporter P-glycoprotein, Stein worked out a simple equation for the “leak-pump” mode of action of the drug efflux pump, P-glycoprotein. [22]

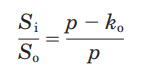
Cell cycle effects of drugs are concentration dependent
[edit]In collaboration with Michael M. Gottesman, Stein demonstrated that high and low concentrations of cytotoxic chemotherapy can provoke two entirely different effects on cell cycle events in NIH3T3 cells despite their intact cell cycle check points: reversible G2/M versus irreversible G1 and S arrest. This experiment demonstrated that understanding clinical drug resistance will require knowledge of the drug levels to which cells are actually exposed [23]
Modeling of P-glycoprotein inhibition
[edit]In collaboration with Dr. Susan Bates, Stein carried out mathematical modeling of P-glycoprotein inhibition in blood samples from patients enrolled on clinical trials of ABCB1 inhibitors. Channel shift values as a function of valspodar (P-glycoprotein inhibitor) blood concentrations were fitted to a simple descending hyperbolic, Michaelis–Menten-type saturation curve, revealing that valspodar is a P-glycoprotein substrate, which is effluxed from the cells. Inhibition appears to plateau above a plasma level of 1000 ng/ml indicating that concentrations were typically in range for inhibition of P-glycoprotein in patient tumors. [24]
Mathematical model of ABCB1 conformational changes
[edit]Together with Igor Roninson and Todd Druley, Stein performed mathematical modeling of conformational changes observed in ABC transporters after substrate binding. [25]
Malaria transport kinetics
[edit]In collaboration with Michael Lanzer, Stein demonstrated that via mutations in the chloroquine resistance transporter (PfCRT), the antimalarial drug chloroquine is transported away from its target, the parasite's digestive vacuole, which does not occur via the wild-type form of PfCRT. [26] They also demonstrated that PfCRT is able to transport diverse antimalarial drugs and that chloroquine and quinine compete for transport via PfCRT in a manner that is consistent with mixed-type inhibition. [27] Other studies showed that PfMDR1 transports drugs, such as chloroquine and quinine, into the digestive vacuole and that mutations in this ABC-transporter reduce drug transport efficiency and, hence, contribute to drug resistance. Their kinetic studies further supported the hypothesis that drug transport via PfCRT and PfMDR1 can incur a fitness cost as these drugs compete with the natural substrate for transport.
An important finding from the joint work with Lanzer, was that – based on transport kinetics – they showed the system is a co-transporter of chloroquine and protons.[28] In addition, work with the Lanzer group led to the identification of a candidate gene for quinine and quinidine resistance.[29]
With his son Moshe Hoshen, together with Hoshen's doctoral supervisor, Hagai Ginsburg, Stein also carried out mathematical modeling of artemisinin treatment of malaria. Hoshen proposed a model in which artemisinin resistance is based on a dormancy stage during which the parasite waits out toxic concentrations of this antimalarial drug.[30] Fundamental to these studies was the mathematical modeling on transport of antimalarial drugs, collaborations with Ginsburg at Hebrew University, that led to a series of joint publications. [31] [32]
Relationship of tumor growth to outcome
[edit]Applying exponential growth kinetics to clinical trial data, Stein derived a set of equations that model tumor growth metrics in patients. These equations have now been applied to over 40,000 patient data sets, in collaboration with Drs. Tito Fojo, Krastan Blagoev, and Susan Bates. [33] [34]
Individual patient data including tumor measurements for most, PSA data for prostate cancer, CA19-9 data for pancreatic cancer, and M-spike measurements, are fit to Stein's series of equations developed and validated that allow determination of regression, d, or growth, g. A series of observations using these equations have been generated – that g correlates with survival, that g is stable while patients are on therapy, that g determines the tumor nadir rather than d, that g for an individual patient can be benchmarked against g for groups of patients, that g can often be determined in a higher fraction of patients on study than a progression endpoint, that g offers a continuous response assessment metric, and that median g for patients enrolled on a clinical trial can be compared across trials, or benchmarked against historical data. This work is poised for use by the pharmaceutical industry in drug development.

On the ages of genes
[edit]In recent years Stein has focused on the phylostratigraphic understanding of gene evolution. [35] [36]
Books
[edit]Science
[edit]- Stein WD. The Movement of Molecules across Cell Membranes. Academic Press 1967.[37]
- Stein WD. Transport and Diffusion Across Cell Membranes. Academic Press 1986.[38]
- Stein WD. Thinking About Biology. Westview Press 1993.[39]
- Stein WD, Litman T. Channels, Carriers, and Pumps: An Introduction to Membrane Transport, 2nd Edition. Academic Press 2015.[40]
Memoir and genealogy
[edit]- Stein WD. The Rolnick Chromosomes: The Global Dispersion of the Rolniks of Lithuania Paperback. CreateSpace Independent Publishing Platform 2014 [1][41]
- Stein WD. Roch's History Of The Morgenstern-Maisel-Atlas Families: A History of the Morgenstern, Maisel and Atlas Families. CreateSpace Independent Publishing Platform 2014 [2][42]
References
[edit]- ^ "Membrane Transport Symposium – Launching Prof. Stein's Book (2015) | The Alexander Silberman Institute of Life Science". www.bio.huji.ac.il.
- ^ "Wilfred D. Stein". Encyclopedia Britannica.
- ^ Stein, W. D. (June 30, 2014). Melanogenesis and the structure of the melanin granule (Thesis) – via wiredspace.wits.ac.za.
- ^ R Morgenstern. Roch’s history of the Morgenstern-Maisel-Atlas Families. Self-published. 2013. ISBN 9 781482 582147
- ^ "A Biophysicist traces his Genealogy | www.telfed.org.il". www.telfed.org.il.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on December 29, 2009. Retrieved November 25, 2019.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ "Wilfred Stein". June 27, 2017.
- ^ "The NIH Catalyst, July–August 1999". nihsearch.cit.nih.gov.
- ^ Barnard, E.A.; Stein, W.D. (December 10, 1959). "The histidine residue in the active centre of ribonuclease". Journal of Molecular Biology. 1 (4–5): 339–349. doi:10.1016/s0022-2836(59)80016-4.
- ^ Stein WD. Intra-protein interactions across a fluid membrane as a model for biological transport. The Journal of general physiology 1969; 54(1): 81–90. doi:10.1085/jgp.54.1.81
- ^ Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972; 175(4023): 720–31. PMID 4333397
- ^ Vereb G et al. Dynamic, yet structured: The cell membrane three decades after the Singer-Nicolson model. Proc Natl Acad Sci USA. 2003; 100: 8053–8. PMID 12832616
- ^ Transport and Diffusion Across Cell Membranes by Wilfred D. Stein. Academic Press, January 1, 1986.
- ^ Karlish SJ, Stein WD. Effects of ATP or phosphate on passive rubidium fluxes mediated by Na-K-ATPase reconstituted into phospholipid vesicles. J Physiol. 1982; 328: 317–31. PubMed PMID 6290647; PubMed Central PMCID: PMC1225660
- ^ Karlish SJ, Stein WD. Cation activation of the pig kidney sodium pump: transmembrane allosteric effects of sodium. J Physiol. 1985 Feb;359:119–49. PubMed PMID 2582111; PubMed Central PMCID: PMC1193368.
- ^ Stein WD, Lieb WR, Karlish SJ, Eilam Y. A model for active transport of sodium and potassium ions as mediated by a tetrameric enzyme. Proc Natl Acad Sci U S A. 1973 Jan;70(1):275–8. PubMed PMID 4265117.
- ^ Bronner F, Stein WD. CaBPr Facilitates Intracellular Diffusion for Ca Pumping in Distal Convoluted Tubule. Am J Physiol 1998; 255(3): F558–62. PMID 2970802 DOI: 10.1152/ajprenal.1988.255.3.F558
- ^ Zeuthen T, Stein WD. Cotransport of salt and water in membrane proteins: membrane proteins as osmotic engines. J Membr Biol. 1994 Feb;137(3):179–95. Review. PubMed PMID 8182729.
- ^ Litman T, Zeuthen T, Skovsgaard T, Stein WD. Structure-activity relationships of P-glycoprotein interacting drugs: kinetic characterization of their effects on ATPase activity. Biochim Biophys Acta. 1997 Aug 22;1361(2):159–68. PubMed PMID 9300797.
- ^ Litman T, Zeuthen T, Skovsgaard T, Stein WD. Competitive, non-competitive and cooperative interactions between substrates of P-glycoprotein as measured by its ATPase activity. Biochim Biophys Acta. 1997 Aug 22;1361(2):169–76. PubMed PMID 9300798.
- ^ Ambudkar SV, Cardarelli CO, Pashinsky I, Stein WD. Relation between the turnover number for vinblastine transport and for vinblastine-stimulated ATP hydrolysis by human P-glycoprotein. J Biol Chem 1997; 272: 21160–6. PMID 9261121
- ^ Litman T, Skovsgaard T, Stein WD. Pumping of drugs by P-glycoprotein: a two-step process? J Pharmacol Exp Ther. 2003 Dec;307(3):846–53. PubMed PMID 14534356.
- ^ Stein WD, Robey R, Cardarelli C, Gottesman MM, Bates SE. Low and high concentrations of the topo II inhibitor daunorubicin in NIH3T3 cells: reversible G2/M versus irreversible G1 and S arrest. Cell Cycle. 2003 Mar-Apr;2(2):134–42. PubMed PMID 12695665.
- ^ Bates SE, Bakke S, Kang M, Robey RW, Zhai S, Thambi P, Chen CC, Patil S, Smith T, Steinberg SM, Merino M, Goldspiel B, Meadows B, Stein WD, Choyke P, Balis F, Figg WD, Fojo T. A phase I/II study of infusional vinblastine with the P-glycoprotein antagonist valspodar (PSC 833) in renal cell carcinoma. Clin Cancer Res. 2004 Jul 15;10(14):4724–33. PubMed PMID 15269145.
- ^ Druley TE, Stein WD, Roninson IB. Analysis of MDR1 P-glycoprotein conformational changes in permeabilized cells using differential immunoreactivity. Biochemistry. 2001 Apr 10;40(14):4312-22. PubMed PMID 11284687.
- ^ Sanchez CP, Rotmann A, Stein WD, Lanzer M. Polymorphisms within PfMDR1 alter the substrate specificity for anti-malarial drugs in Plasmodium falciparum. Mol Microbiol. 2008 Nov;70(4):786–98. doi: 10.1111/j.1365-2958.2008.06413.x. Epub 2008 Aug 18. PubMed PMID 18713316.
- ^ Bellanca S, Summers RL, Meyrath M, Dave A, Nash MN, Dittmer M, Sanchez CP, Stein WD, Martin RE, Lanzer M. Multiple drugs compete for transport via the Plasmodium falciparum chloroquine resistance transporter at distinct but interdependent sites. J Biol Chem. 2014 Dec 26;289(52):36336–51. PubMed PMID 25378409.
- ^ Sanchez CP1, Stein WD, Lanzer M. Is PfCRT a channel or a carrier? Two competing models explaining chloroquine resistance in Plasmodium falciparum. Trends Parasitol. 2007; 23: 332–9. PMID 17493873
- ^ Sanchez CP, Liu CH, Mayer S, Nurhasanah A, Cyrklaff M, Mu J, Ferdig MT, Stein WD, Lanzer M. A HECT Ubiquitin-Protein Ligase as a Novel Candidate Gene for Altered Quinine and Quinidine Responses in Plasmodium falciparum. PLoS Genet 2014; 10(5): e1004382. PMCID: PMC4022464. PMID 24830312
- ^ Hoshen MB, Na-Bangchang K, Stein WD, Ginsburg H. Mathematical modelling of the chemotherapy of Plasmodium falciparum malaria with artesunate: postulation of 'dormancy', a partial cytostatic effect of the drug, and its implication for treatment regimens. Parasitology. 2000 Sep;121 (Pt 3):237–46. PMID 11085244.
- ^ Ginsburg H, Stein WD. Biophysical analysis of novel transport pathways induced in red blood cell membranes. J Membr Biol. 1987 96(1):1–10.
- ^ Ginsburg H, Stein WD. Kinetic modelling of chloroquine uptake by malaria-infected erythrocytes. Assessment of the factors that may determine drug resistance. Biochem Pharmacol. 1991 May 15;41(10):1463–70.
- ^ Stein WD, Figg WD, Dahut W, Stein AD, Hoshen MB, Price D, Bates SE, Fojo T. Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncologist. 2008 Oct;13(10):1046-54. PubMed PMID 18838440.
- ^ Stein WD, Huang H, Menefee M, Edgerly M, Kotz H, Dwyer A, Yang J, Bates SE. Other paradigms: growth rate constants and tumor burden determined using computed tomography data correlate strongly with the overall survival of patients with renal cell carcinoma. Cancer J. 2009 Sep-Oct;15(5):441-7. PubMed PMID 19826366.
- ^ Litman T, Stein WD. Obtaining estimates for the ages of all the protein-coding genes and most of the ontology-identified noncoding genes of the human genome, assigned to 19 phylostrata. Semin Oncol. 2019 Feb;46(1):3–9. PubMed PMID 30558821.
- ^ Stein WD. The ages of the cancer-associated genes. Semin Oncol. 2019 Feb;46(1):10–18. PubMed PMID 30554805.
- ^ Stein WD. The Movement of Molecules across Cell Membranes. Academic Press 1967. ISBN 9780323152679
- ^ Stein WD. Transport & Diffusion Across Cell Membranes. Academic Press 1986. ISBN 0126646600
- ^ Stein WD. Thinking About Biology. Westview Press 1993.
- ^ Stein WD, Litman T. Channels, Carriers, and Pumps: An Introduction to Membrane Transport, 2nd Edition. Academic Press 2015.
- ^ Stein WD. The Rolnick Chromosomes: The Global Dispersion of the Rolniks of Lithuania Paperback. CreateSpace Independent Publishing Platform 2014
- ^ Stein WD. Roch's History Of The Morgenstern-Maisel-Atlas Families: A History of the Morgenstern, Maisel and Atlas Families. CreateSpace Independent Publishing Platform 2014
