User:Cleanthis/draft confisomer
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds.[1] Such isomers are generally referred to as conformational isomers or conformers and specifically as rotamers[2] when they differ by rotation about only one single bond. Conformational isomers are thus distinct from the other classes of stereoisomers for which interconvertion necessarily involves breaking and reforming of chemical bonds.
Types of conformational isomerism
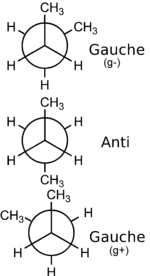
[edit]The rotational barrier, or barrier to rotation, is the activation energy required to convert from one rotamer to another rotamer. The existence of more than one conformation, usually with different energies, is due to hindered rotation about sp3 hybridised σ bonds. The comparative stabilities of different conformers of a molecule are usually explained through differences in a combination of steric repulsion and electronic effects. A simplified example is that of a butane molecule viewed in the Newman projection shown (viewed down the central C2-C3 bond) with relative rotations of C1 and C4 illustrated. The two gauche conformers of n-butane are enantiomers of equal energy content and have a dihedral angle of 60°.The anti conformer has a planar geometry so the dihedral angle is 180°. The three eclipsed conformations with dihedral angles of 0°,120° and 270° are only unstable transitional structures in the interconversion of rotamers and are not considered to be rotamers themselves.
Important examples of conformational isomerism include:
- Linear alkane conformations with staggered, eclipsed and gauche conformers, and
- Ring conformation
- Cyclohexane conformations with chair and boat conformers.
- Carbohydrates
- Steroids
- Atropisomerism- due to restricted rotation about a bond, a molecule can become chiral
- Folding of molecules, where some shapes are stable and functional, but others are not.
Equilibrium Population of Conformers
[edit]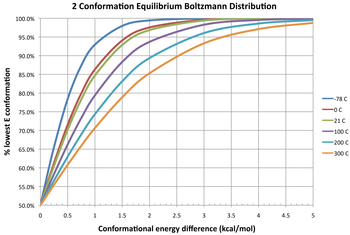
The population of different conformers follows a Boltzmann distribution:
The left hand side is the equilibrium ratio of conformer i to the total. is the relative energy of the i-th conformer from the minimum energy conformer. R is the molar ideal gas constant equal to 8.31 J/(mol·K) and T is the temperature in kelvins (K). The denominator of the right side is the partition function.
Isolation or observation of the conformational isomers
[edit]- Atropoisomers can be quite stable molecular structures and depending on the steric bulk of the substituents occupying the ortho positions of the biphenylic system they inter convert very slowly at ordinary temperatures so that the usual laboratory techniques can be applied for their isolation and characterization (chromatographies,crystallization, NMR spectroscopy etc ).They were the first conformational isomers to be identified and studied[3].
- In simple cyclohexane derivatives ,at room temperature, the two chair conformers inter convert with rates typically of the order of 105ring flips/sec[3].Only by working at very low temperature can this interconversion be slowed down enough to allow for the two components to be separated and maintained long enough to be identified.Cyclohexyl chloride for instance,cooled in acetone solution at-1000C deposits crystals of the equatorial conformer while the mother liquor contains the axial conformer[4].When the isolated crystals are allowed to warm back to a higher temperature equilibrium is restored and the sample is found to contain again both axial and equatorial conformers.
- IR spectroscopy is ordinarily used to measure conformer ratios. The two conformers of cyclohexyl bromide,for example, have stretching frequencies for the C-Br bond differing by almost 50 1/cm which allows for them to be observed separately[5].
- NMR spectroscopic examination of compounds consisting of fast equilibrating conformers ,ordinarily fails to record signals that can be attributed specifically to each particular conformer. When such spectra are recorded at room temperature the frequency of the signal from each particular nucleus is the weighted average of the frequencies corresponding to each of the conformers .On the contrary ,recording the NMR spectrum at a sufficiently low temperature ("freezing the structure") produces a spectrum that is the simple sum of the component spectra ,one for each conformer;under these conditions the conformers behave like an ordinary mixture of compounds.[Note 1]
Consequences
[edit]If the eclipsed conformations of an isomer have high enough potentials, they may prevent rotation of substituents to different staggered conformations at sufficiently low energy levels. This will result in a racemic mixture of conformations that may or may not have different reactivities in situations such as enzymatic reactions in which molecular shape is usually a key factor of operation.
Conformer dependent reactions
[edit]The E2 elimination mechanism relies on the base- or acid-attacked substituent being in an antiperiplanar configuration along a bond with respect to the leaving group. This prerequisite for reaction is important in understanding organic elimination reaction pathways, especially those involving halogenated cyclic alkanes such as cyclohexanes. Two adjacent substituents on a cyclic alkane can only undergo an E2 elimination if they are both axial to the ring and hence antiperiplanar. A combination of axial and equatorial substituents cannot react through an E2 mechanism, though ring flips (with associated reconformation) may allow reactions to occur if they are not precluded by an energy barrier or steric lock through isopropyl or larger substituents.
Conditions
[edit]Conformational isomerism only occurs around single bonds because double or triple bonds have one or two pi bonds that prevent rotation about the longitudinal axis. Conformers sufficiently constrained to exhibit measurable isomerism are unique from various flavours of stereoisomers in the fact that changes in stereochemistry are independent from any mechanism and instead rely only on molecular energy.
Protein Rotamer Libraries
[edit]A rotamer library is a collection of rotamers for each residue type in proteins with side-chain degrees of freedom. Rotamer libraries usually contain information about both conformation and frequency of a certain conformation. Often libraries will also contain information about the variance about dihedral angle means or modes, which can be used in sampling.[6]
Side-chain dihedral angles are not evenly distributed, but for most side chain types, the angles occur in tight clusters around certain values. Rotamer libraries therefore are usually derived from statistical analysis of side-chain conformations in known structures of proteins by clustering observed conformations or by dividing dihedral angle space into bins, and determining an average conformation in each bin. This division is usually on physical-chemical grounds, as in the divisions for rotation about sp3-sp3 bonds into three 120° bins centered on each staggered conformation (60°, 180°, -60°).
Rotamer libraries can be backbone-independent, secondary-structure-dependent, or backbone-dependent. The distinctions are made depending on whether the dihedral angles for the rotamers and/or their frequencies depend on the local backbone conformation or not. Backbone-independent rotamer libraries make no reference to backbone conformation, and are calculated from all available side chains of a certain type.[7] Secondary-structure-dependent libraries present different dihedral angles and/or rotamer frequencies for -helix, -sheet, or coil secondary structures. Backbone-dependent rotamer libraries present conformations and/or frequencies dependent on the local backbone conformation as defined by the backbone dihedral angles and , regardless of secondary structure.[8] Finally, a variant on backbone-dependent rotamer libraries exists in the form of position-specific rotamers, those defined by a fragment usually of 5 amino acids in length, where the central residue’s side chain conformation is examined.
See also
[edit]Notes
[edit]- ^ This strong temperature dependence of the NMR spectrum can be crudely explained by referring to the blurring seen in photographs of fast moving objects;the radio frequencies involved in the NMR experiments are not as high as those of the IR radiation and consequently any movement within the molecule must be suppressed by cooling the sample to a temperature as low as is practically possible in order to diminish "blurring" of the spectrum.The frequencies utilized in an ordinary (200 MHz) 1HNMR spectrometer are five orders of magnitude lower than those of hydrogen streching vibrations at around 3000 1/cm.
.
References
[edit]- ^ IUPAC definition of a conformer.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1996) "Rotamer". doi:10.1351/goldbook.F02520
- ^ a b Eliel E L Stereochemistry Of Organic Compounds,Cbs Publishers,2003,ISBN 9814126624
- ^ Jensen experiment
- ^ eliel book
- ^ Dunbrack, RL (2002). "Rotamer Libraries in the 21st Century". Curr. Opin. Struct. Biol. 12 (4): 431-440. doi:10.1016/S0959-440X(02)00344-5. PMID 12163064.
- ^ Richardson Rotamer Libraries
- ^ Dunbrack Rotamer Libraries
Category:Stereochemistry Category:Physical organic chemistry Category:Isomerism







