User:ChemistBoyBest/sandbox
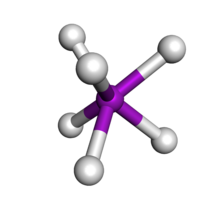

Molybdenum hydride VI (chemical formula MoH6) is a unstable volatile gas. Because of the high radius of molybdenum it quickly breaks down when it is not under pressure.
Structure
[edit]It is known to have a distorted trigonal prism structure. This structure follows the VSEPR Theory.
Properties
[edit]It is one of the components in which molybdenum has the maximum oxidation number +6 and having similar properties to H2Mo or H2S. It can be used as a oxidation agent in redox reactions.
Molybdenum hydride VI is an acidic hydride , because it manifests amphoteric properties. In reaction with most alkaline bases forms stable complexes.
Synthesis
[edit]Laser-ablated molybdenum atoms react with hydrogen gas upon condensation in excess argon and neon. Molybdenum gets oxidized by hydrogen till the maximum oxidation grade.
2Mo + H2 (hv)→ 2MoH (+∆H)
2MoH + H2 (hv)→ 2H2Mo (+∆H)
H2Mo + H2 (hv)→ MoH4 (+∆H)
H2 + MoH4 (hv)→ MoH6 (+∆H)
