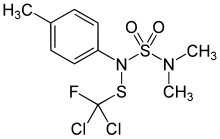
Tolylfluanid
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-{[Dichloro(fluoro)methyl]sulfanyl}-N′,N′-dimethyl-N-(4-methylphenyl)sulfuric diamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.010.898 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H13Cl2FN2O2S2 | |
| Molar mass | 347.244 g/mol |
| Appearance | colourless, odourless crystals |
| Density | 1.52 g/cm3 |
| Melting point | 93°C |
| Boiling point | Decomposes on distillation |
| water, 0.9 mg/L at 20°C. Miscible in all proportions with acetone, ethanol, ethyl acetate, methylene chloride | |
| Vapor pressure | <1.3 mPa (20°C) |
| Related compounds | |
Related compounds
|
Dichlofluanid |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
LC50 (median concentration)
|
0.02-0.3 mg/L |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tolylfluanid is an organic chemical compound that is used as an active ingredient in fungicides and wood preservatives.
Synthesis
[edit]The synthesis of tolylfluanid begins with the reaction of dimethylamine and sulfuryl chloride. The product further reacts with p-toluidine and dichlorofluoromethanesulfenyl chloride to yield the final product.[1]
Use
[edit]Tolylfluanid is used on fruit and ornamental plants against gray mold (Botrytis), against late blight on tomatoes and against powdery mildew on cucumbers.
Environmental behavior
[edit]Tolylfluanid hydrolyzes slowly in acidic conditions. The half-life is shorter when the pH is high; at pH = 7, it is at least 2 days. In aerobic media (pH = 7.7-8.0), tolylfluanid hydrolytically and microbially decomposes to N,N-dimethyl-N-(4-methylphenyl) sulfamide (DMST) and dimethylsulfamide. After 14 days, tolylfluanid is generally considered to have degraded. The half-life of DMST is 50-70 days. [2]
Absorption, metabolism and excretion
[edit]Tolylfluanid is rapidly and almost completely absorbed in the gastrointestinal tract. The highest concentrations are found in the blood, lungs, liver, kidneys, spleen and thyroid gland. 99% is excreted in the urine within two days, although there is some accumulation in the thyroid gland. [2]
References
[edit]- ^ Thomas A. Unger (1996). Pesticide Synthesis Handbook. William Andrew. p. 985. ISBN 0-8155-1853-6.
- ^ a b Svensk Chemicals Inspection : Tolylfluanid (PDF). Archived from the original (PDF) on 2007-09-27.
