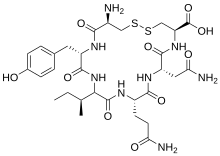
Tocinoic acid
Appearance
 | |
| Clinical data | |
|---|---|
| Other names | TOC; 7-de-Pro-3-de-Leu-3-de-GlyNH2-deoxytocin |
| Drug class | Oxytocin receptor antagonist |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C30H44N8O10S2 |
| Molar mass | 740.85 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tocinoic acid is a peptide oxytocin receptor antagonist which is used in scientific research.[1] Intracerebroventricular injection of the drug has been found to block the prosocial behavior induced by the serotonin releasing agent MDMA without affecting baseline social behavior in animals.[2][3][4][1] Similar findings have been made for the non-peptide selective oxytocin receptor antagonist L-368899.[2][5] However, in other studies, other oxytocin receptor antagonists have been ineffective in blocking MDMA-induced prosocial behavior.[1][6][7] The reasons for these discrepancies are unclear.[2][1]
See also
[edit]References
[edit]- ^ a b c d Dunlap LE, Andrews AM, Olson DE (October 2018). "Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine". ACS Chemical Neuroscience. 9 (10): 2408–2427. doi:10.1021/acschemneuro.8b00155. PMC 6197894. PMID 30001118.
- ^ a b c Wronikowska-Denysiuk O, Mrozek W, Budzyńska B (February 2023). "The Role of Oxytocin and Vasopressin in Drug-Induced Reward-Implications for Social and Non-Social Factors". Biomolecules. 13 (3): 405. doi:10.3390/biom13030405. PMC 10046619. PMID 36979340.
- ^ Carson DS, Guastella AJ, Taylor ER, McGregor IS (March 2013). "A brief history of oxytocin and its role in modulating psychostimulant effects". Journal of Psychopharmacology. 27 (3): 231–247. doi:10.1177/0269881112473788. PMID 23348754.
- ^ McGregor IS, Callaghan PD, Hunt GE (May 2008). "From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use?". British Journal of Pharmacology. 154 (2): 358–368. doi:10.1038/bjp.2008.132. PMC 2442436. PMID 18475254.
- ^ Kuteykin-Teplyakov K, Maldonado R (November 2014). "Looking for prosocial genes: ITRAQ analysis of proteins involved in MDMA-induced sociability in mice". European Neuropsychopharmacology. 24 (11): 1773–1783. doi:10.1016/j.euroneuro.2014.08.007. hdl:10230/23309. PMID 25241352.
- ^ Kamilar-Britt P, Bedi G (October 2015). "The prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA): Controlled studies in humans and laboratory animals". Neuroscience and Biobehavioral Reviews. 57: 433–446. doi:10.1016/j.neubiorev.2015.08.016. PMC 4678620. PMID 26408071.
- ^ Heifets BD, Salgado JS, Taylor MD, Hoerbelt P, Cardozo Pinto DF, Steinberg EE, et al. (December 2019). "Distinct neural mechanisms for the prosocial and rewarding properties of MDMA". Science Translational Medicine. 11 (522). doi:10.1126/scitranslmed.aaw6435. PMC 7123941. PMID 31826983.
