Talk:Diborane(4)
Appearance
| This article is rated Stub-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Does this compound exist? And if so what is is its structure?
[edit]The claim that this compound has never been isolated was removed. This was probably a mistake. A structural IUPAC name has been assigned but there is no physical data to support this. All of the evidence seems to point to this being a non-existent compound.Axiosaurus (talk) 13:46, 16 October 2013 (UTC)
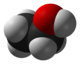
Inage file- any physiacle evidence for the ethene structure?
[edit]Is there any evidence for the structure of the compound- computational papers indicate a bridged structure- diborane with two terminal H removed- rather than an ethene like structure. Axiosaurus (talk) 07:48, 23 June 2014 (UTC)
- The article says "the results obtained by photoionization mass spectrometry are consistent with a doubly bridged structure" with a cite but I don't have access to the full J.Chem.Phys. paper to know what that evidence is (or how strongly it contradicts other possibilities. However, I do see several computational papers on the non-bridged structure predicting B–B dihedral of ~90° (exactly unlike ethene). That's the same angle seems like it would lead to the bridged form pretty easily. SciFinder doesn't have any more-recent experimental papers that cite the that J.Chem.Phys. article. DMacks (talk) 09:17, 23 June 2014 (UTC)
- Unless there's strong evidence for the traditional-bonding model, the image in the article should be replaced. The current one is exported from ChEBI, which does not cite any other structural-analysis references (I would assume it's machine-generated/predicted from the formula). DMacks (talk) 09:26, 23 June 2014 (UTC)
- FWIW: I added the image yesterday just because it is eponymous and it shows some structure. I cannot claim any more wisdom in this. -DePiep (talk) 14:02, 23 June 2014 (UTC)
- Unless there's strong evidence for the traditional-bonding model, the image in the article should be replaced. The current one is exported from ChEBI, which does not cite any other structural-analysis references (I would assume it's machine-generated/predicted from the formula). DMacks (talk) 09:26, 23 June 2014 (UTC)
- I'll give an excerpt from the paper relating to the evidence. Plasmic Physics (talk) 22:17, 23 June 2014 (UTC)
- "Our goal in the experimental program described below was to prepare B
2H
4 and to obtain its photoion yield curve. The shape of this curve could provide clues about the structure of B
2H
4 which was being detected. Furthermore, the experimental adiabatic and vertical ionization potentials could be compared with the values predicted by ab initio calculations, thereby providing another test of the structure and energetics of B
2H
4 and B
2H+
4... A B
2H
4 species has been produced by the successive hydrogen abstraction reactions of F atoms with B
2H
6. The photoion yield curve of this species yields an adiabatic ionization potential of 9.70±0.02 eV, a vertical ionization potential of ~10.4 eV, and some step structure indicative of a vibrational progression, with (ω=1300+100 cm−1. These results are in essential agreement with ab initio calculations, if the B
2H
4 species detected has a doubly bridged, C2v structure." Moreover, the structure is not planar, but puckered instead. Plasmic Physics (talk) 23:00, 23 June 2014 (UTC)
- I uploaded a new image based on the cited refs. DMacks (talk) 15:52, 20 August 2015 (UTC)