Purinergic signalling
| Part of a series on |
| Purinergic signalling |
|---|
 |
| Concepts |
| Membrane transporters |
Purinergic signalling (or signaling: see American and British English differences) is a form of extracellular signalling mediated by purine nucleotides and nucleosides such as adenosine and ATP. It involves the activation of purinergic receptors in the cell and/or in nearby cells, thereby regulating cellular functions.[1]
It was proposed after Adenosine triphosphate (ATP) was identified in 1970 as the transmitter responsible for non-adrenergic, noncholinergic neurotransmission. Nowadays is it known that ATP acts a cotransmitter in most, if not all, nerves in the central and peripheral nervous system.[2]
Receptors for adenosine (called P1) and for ATP and ADP (called P2) were distinguished in 1978. Later, the P2 receptors were subdivided into P2X and P2Y families based on their different mechanisms. In the early 1990s, when the receptors to purines and pyrimidines were cloned and characterized, numerous subtypes of P1 and P2 receptors were discovered.[3]
The purinergic signalling complex of a cell is sometimes referred to as the “purinome”.[4]
Background
[edit]Evolutionary origins
[edit]
Purinergic receptors, represented by several families, are among the most abundant receptors in living organisms and appeared early in evolution.[6]
Among invertebrates, the purinergic signalling system has been found in bacteria, amoeba, ciliates, algae, fungi, anemones, ctenophores, platyhelminthes, nematodes, crustacea, molluscs, annelids, echinoderms, and insects.[7] In green plants, extracellular ATP and other nucleotides induce an increase in the cytosolic concentration of calcium ions, in addition to other downstream changes that influence plant growth and modulate responses to stimuli.[8] In 2014, the first purinergic receptor in plants, DORN1, was discovered.[9]
The primitive P2X receptors of unicellular organisms often share low sequence similarity with those in mammals, yet they still retain micromolar sensitivity to ATP. The evolution of this receptor class is estimated to have occurred over a billion years ago.[10]
Molecular mechanisms
[edit]Generally speaking, all cells have the ability to release nucleotides. In neuronal and neuroendocrinal cells, this mostly occurs via regulated exocytosis.[1] Released nucleotides can be hydrolyzed extracellularly by a variety of cell surface-located enzymes referred to as ectonucleotidases. The purinergic signalling system consists of transporters, enzymes and receptors responsible for the synthesis, release, action, and extracellular inactivation of (primarily) ATP and its extracellular breakdown product adenosine.[11] The signalling effects of uridine triphosphate (UTP) and uridine diphosphate (UDP) are generally comparable to those of ATP.[12]
Purinergic receptors
[edit]
Purinergic receptors are specific classes of membrane receptors that mediate various physiological functions such as the relaxation of gut smooth muscle, as a response to the release of ATP or adenosine. There are three known distinct classes of purinergic receptors, known as P1, P2X, and P2Y receptors. Cell signalling events initiated by P1 and P2Y receptors have opposing effects in biological systems.[13]
| Name | Activation | Class |
| P1 receptors | adenosine | G protein-coupled receptors |
| P2Y receptors | nucleotides | G protein-coupled receptors |
| P2X receptors | ATP | ligand-gated ion channel |
Nucleoside transporters
[edit]Nucleoside transporters (NTs) are a group of membrane transport proteins which transport nucleoside substrates including adenosine across the membranes of cells and/or vesicles. NTs are considered to be evolutionarily ancient membrane proteins and are found in many different forms of life.[14] There are two types of NTs:
- Concentrative nucleoside transporters (CNTs): Na+-dependent symporters[14]
- Equilibrative nucleoside transporters (ENTs): Na+-independent passive transporters[14]
The extracellular concentration of adenosine can be regulated by NTs, possibly in the form of a feedback loop connecting receptor signaling with transporter function.[14]
Ectonucleotidases
[edit]Released nucleotides can be hydrolyzed extracellularly by a variety of cell surface-located enzymes referred to as ectonucleotidases that control purinergic signalling. Extracellular nucleoside triphosphates and diphosphates are substrates of the ectonucleoside triphosphate diphosphohydrolases (E-NTPDases), the ectonucleotide pyrophosphatase/phosphodiesterases (E-NPPs) and alkaline phosphatases (APs). Extracellular AMP is hydrolyzed to adenosine by ecto-5'-nucleotidase (eN) as well as by APs. In any case, the final product of the hydrolysis cascade is the nucleoside.[15][16]
Pannexins
[edit]The Pannexin-1 channel (PANX1) is an integral component of the P2X/P2Y purinergic signaling pathway and the key contributor to pathophysiological ATP release.[17] For example, the PANX1 channel, along with ATP, purinergic receptors, and ectonucleotidases, contribute to several feedback loops during the inflammatory response.[18]
Purinergic signalling in humans
[edit]Circulatory system
[edit]In the human heart, adenosine functions as an autacoid in the regulation of various cardiac functions such as heart rate, contractility, and coronary flow. There are currently four types of adenosine receptors found in the heart.[19] After binding onto a specific purinergic receptor, adenosine causes a negative chronotropic effect due to its influence on cardiac pacemakers. It also causes a negative dromotropic effect through the inhibition of AV-nodal conduction.[20] From the 1980s onwards, these effects of adenosine have been used in the treatment of patients with supraventricular tachycardia.[21]
The regulation of vascular tone in the endothelium of blood vessels is mediated by purinergic signalling. A decreased concentration of oxygen releases ATP from erythrocytes, triggering a propagated calcium wave in the endothelial layer of blood vessels and a subsequent production of nitric oxide that results in vasodilation.[22][23]
During the blood clotting process, adenosine diphosphate (ADP) plays a crucial role in the activation and recruitment of platelets and also ensures the structural integrity of thrombi. These effects are modulated by the P2RY1 and the P2Y12 receptors. The P2RY1 receptor is responsible for shape change in platelets, increased intracellular calcium levels and transient platelet aggregation, while the P2Y12 receptor is responsible for sustained platelet aggregation through the inhibition of adenylate cyclase and a corresponding decrease in cyclic adenosine monophosphate (cAMP) levels. The activation of both purinergic receptors is necessary to achieve sustained hemostasis.[24][25]
Digestive system
[edit]In the liver, ATP is constantly released during homeostasis and its signalling via P2 receptors influences bile secretion as well as liver metabolism and regeneration.[26] P2Y receptors in the enteric nervous system and at intestinal neuromuscular junctions modulate intestinal secretion and motility.[27]
Endocrine system
[edit]Cells of the pituitary gland secrete ATP, which acts on P2Y and P2X purinoreceptors.[28]
Immune system
[edit]
Autocrine purinergic signalling is an important checkpoint in the activation of white blood cells. These mechanisms either enhance or inhibit cell activation based on the purinergic receptors involved, allowing cells to adjust their functional responses initiated by extracellular environmental cues.[29]
Like most immunomodulating agents, ATP can act either as an immunosuppressive or an immunostimulatory factor, depending on the cytokine microenvironment and the type of cell receptor.[30] In white blood cells such as macrophages, dendritic cells, lymphocytes, eosinophils, and mast cells, purinergic signalling plays a pathophysiological role in calcium mobilization, actin polymerization, release of mediators, cell maturation, cytotoxicity, and apoptosis.[31] Large increases in extracellular ATP that are associated with cell death serve as a "danger signal" in the inflammatory processes.[32]
In neutrophils, tissue adenosine can either activate or inhibit various neutrophil functions, depending on the inflammatory microenvironment, the expression of adenosine receptors on the neutrophil, and the affinity of these receptors for adenosine. Micromolar concentrations of adenosine activate A2A and A2B receptors. This inhibits the release of granules and prevents oxidative burst. On the other hand, nanomolar concentrations of adenosine activate A1 and A3 receptors, resulting in neutrophilic chemotaxis towards inflammatory stimuli. The release of ATP and an autocrine feedback through P2RY2 and A3 receptors are signal amplifiers.[33][34] Hypoxia-inducible factors also influence adenosine signalling.[21]
Nervous system
[edit]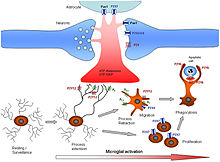
In the central nervous system (CNS), ATP is released from synaptic terminals and binds to a plethora of ionotropic and metabotropic receptors. It has an excitatory effect on neurones, and acts as a mediator in neuronal–glial communications.[35] Both adenosine and ATP induce astrocyte cell proliferation. In microglia, P2X and P2Y receptors are expressed. The P2Y6 receptor, which is primarily mediated by uridine diphosphate (UDP), plays a significant role in microglial phagoptosis, while the P2Y12 receptor functions as a specialized pattern recognition receptor. P2RX4 receptors are involved in the CNS mediation of neuropathic pain.[36]
In the peripheral nervous system, Schwann cells respond to nerve stimulation and modulate the release of neurotransmitters through mechanisms involving ATP and adenosine signalling.[37] In the retina and the olfactory bulb, ATP is released by neurons to evoke transient calcium signals in several glial cells such as Muller glia and astrocytes. This influences various homeostatic processes of the nervous tissue including volume regulation and the control of blood flow. Although purinergic signaling has been connected to pathological processes in the context of neuron-glia communication, it has been revealed, that this is also very important under physiological conditions. Neurons possess specialised sites on their cell bodies, through which they release ATP (and other substances), reflecting their "well-being". Microglial processes specifically recognize these purinergic somatic-junctions, and monitor neuronal functions by sensing purine nucleotides via their P2Y12-receptors. In case of neuronal overactivation or injury, microglial processes respond with an increased coverage of neuronal cell bodies, and exert robust neuroprotective effects.[38] These purinergic somatic-junctions have also been shown to be important for microglia to control neuronal development. [39] Calcium signaling evoked by purinergic receptors contributes to the processing of sensory information.[40]
During neurogenesis and in early brain development, ectonucleotidases often downregulate purinergic signalling in order to prevent the uncontrolled growth of progenitor cells and to establish a suitable environment for neuronal differentiation.[41]
Purinergic signalling, and in particular tissue-injury induced ATP-release is very important for the rapid and robust phenotype changes of microglia. [42]
Renal system
[edit]In the kidneys, the glomerular filtration rate (GFR) is regulated by several mechanisms including tubuloglomerular feedback (TGF), in which an increased distal tubular sodium chloride concentration causes a basolateral release of ATP from the macula densa cells. This initiates a cascade of events that ultimately brings GFR to an appropriate level.[43][44]
Respiratory system
[edit]ATP and adenosine are crucial regulators of mucociliary clearance.[45] The secretion of mucin involves P2RY2 receptors found on the apical membrane of goblet cells.[45] Extracellular ATP signals acting on glial cells and the neurons of the respiratory rhythm generator contribute to the regulation of breathing.[46]
Skeletal system
[edit]In the human skeleton, nearly all P2Y and P2X receptors have been found in osteoblasts and osteoclasts. These receptors enable the regulation of multiple processes such as cell proliferation, differentiation, function, and death.[47] The activation of the adenosine A1 receptor is required for osteoclast differentiation and function, whereas the activation of the adenosine A2A receptor inhibits osteoclast function. The other three adenosine receptors are involved in bone formation.[48]
Pathological aspects
[edit]Alzheimer's disease
[edit]In Alzheimer's disease (AD), the expression of A1 and A2A receptors in the frontal cortex of the human brain is increased, while the expression of A1 receptors in the outer layers of hippocampal dentate gyrus is decreased.[41]
Asthma
[edit]In the airways of patients with asthma, the expression of adenosine receptors is upregulated. Adenosine receptors affect bronchial reactivity, endothelial permeability, fibrosis, angiogenesis and mucus production.[49]
Bone diseases
[edit]Purinergic signalling is involved in the pathophysiology of several bone and cartilage diseases such as osteoarthritis, rheumatoid arthritis, and osteoporosis.[50] Single-nucleotide polymorphisms (SNPs) in the P2RX7 receptor gene are associated with an increased risk of bone fracture.[47]
Cancer
[edit]The P2RX7 receptor is overexpressed in most malignant tumors.[51] The expression of the adenosine A2A receptor on endothelial cells is upregulated in the early stages of human lung cancer.[52]
Cardiovascular diseases
[edit]Formation of foam cells is inhibited by adenosine A2A receptors.[53]
Chronic obstructive pulmonary disease
[edit]Abnormal levels of ATP and adenosine are present in the airways of patients with chronic obstructive pulmonary disease.[54][55]
Erectile disorders
[edit]The release of ATP increases adenosine levels and activates nitric oxide synthase, both of which induces the relaxation of the corpus cavernosum penis. In male patients with vasculogenic impotence, dysfunctional adenosine A2B receptors are associated with the resistance of the corpus cavernosum to adenosine. On the other hand, excess adenosine in penile tissue contributes to priapism.[56][57]
Fibrosis
[edit]The bronchoalveolar lavage (BAL) fluid of patients with idiopathic pulmonary fibrosis contains a higher concentration of ATP than that of control subjects.[58] Persistently elevated concentrations of adenosine beyond the acute-injury phase leads to fibrotic remodelling.[59] Extracellular purines modulate fibroblast proliferation by binding onto adenosine receptors and P2 receptors to influence tissue structure and pathologic remodeling.[58]
Graft-versus-host disease
[edit]Following tissue injury in patients with Graft-versus-host disease (GVHD), ATP is released into the peritoneal fluid. It binds onto the P2RX7 receptors of host antigen-presenting cells (APCs) and activates the inflammasomes. As a result, the expression of co-stimulatory molecules by APCs is upregulated. The inhibition of the P2X7 receptor increases the number of regulatory T cells and decreases the incidence of acute GVHD.[60]
Therapeutic interventions
[edit]Current
[edit]
- Acupuncture
Mechanical deformation of the skin by acupuncture needles appears to result in the release of adenosine.[62][63] A 2014 Nature Reviews Cancer review article found that the key mouse studies that suggested acupuncture relieves pain via the local release of adenosine, which then triggered close-by A1 receptors "caused more tissue damage and inflammation relative to the size of the animal in mice than in humans, such studies unnecessarily muddled a finding that local inflammation can result in the local release of adenosine with analgesic effect."[64] The anti-nociceptive effect of acupuncture may be mediated by the adenosine A1 receptor.[65][66][67] Electroacupuncture may inhibit pain by the activation of a variety of bioactive chemicals through peripheral, spinal, and supraspinal mechanisms of the nervous system.[68]
- Anti-inflammatory drugs
Methotrexate, which has strong anti-inflammatory properties, inhibits the action of dihydrofolate reductase, leading to an accumulation of adenosine. On the other hand, the adenosine-receptor antagonist caffeine reverses the anti-inflammatory effects of methotrexate.[69]
- Anti-platelet drugs
Many anti-platelet drugs such as Prasugrel, Ticagrelor, and Ticlopidine are adenosine diphosphate (ADP) receptor inhibitors. Before the expiry of its patent, the P2Y12 receptor antagonist Clopidogrel (trade name: Plavix) was the second most prescribed drug in the world. In 2010 alone, it generated over US$9 billion in global sales.[70]
- Bronchodilators
Theophylline was originally used as a bronchodilator, although its usage has declined due to several side effects such as seizures and cardiac arrhythmias caused by adenosine A1 receptor antagonism.[71]
- Herbal medicine
Several herbs used in Traditional Chinese medicine contain drug compounds that are antagonists of P2X purinoreceptors.[72] The following table provides an overview of these drug compounds and their interaction with purinergic receptors.
| Herb | Drug compound | Physiologic effects on purinergic receptors |
| Many |
| |
| Ligusticum wallichii |
| |
| Kudzu | ||
| Rheum officinale | ||
| Rhubarb |
- Vasodilators
Regadenoson, a vasodilator which acts on the adenosine A2A receptor, was approved by the United States Food and Drug Administration in 2008 and is currently widely used in the field of cardiology.[80][81] Both adenosine and dipyridamole, which act on the A2A receptor, are used in myocardial perfusion imaging.[82]
Proposed
[edit]Purinergic signalling is an important regulatory mechanism in a wide range of inflammatory diseases. It is understood that shifting the balance between purinergic P1 and P2 signalling is an emerging therapeutic concept that aims to dampen pathologic inflammation and promote healing.[13] The following list of proposed medications is based on the workings of the purinergic signalling system:
- Diquafosol - Agonist of the P2Y2 receptor used in the treatment of dry eye disease.[83]
- Istradefylline - Antagonist of the adenosine A2A receptor, used in the treatment of Parkinson's disease as an adjunct to L-DOPA.[84]
History
[edit]The earliest reports of purinergic signalling date back to 1929, when the Hungarian physiologist Albert Szent-Györgyi observed that purified adenine compounds produced a temporary reduction in heart rate when injected into animals.[13][85]
In the 1960s, the classical view of autonomic smooth muscle control was based upon Dale's principle, which asserts that each nerve cell can synthesize, store, and release only one neurotransmitter. It was therefore assumed that a sympathetic neuron releases noradrenaline only, while an antagonistic parasympathetic neuron releases acetylcholine only. Although the concept of cotransmission gradually gained acceptance in the 1980s, the belief that a single neuron acts via a single type of neurotransmitter continued to dominate the field of neurotransmission throughout the 1970s.[86]
Beginning in 1972, Geoffrey Burnstock ignited decades of controversy after he proposed the existence of a non-adrenergic, non-cholinergic (NANC) neurotransmitter, which he identified as ATP after observing the cellular responses in a number of systems exposed to the presence of cholinergic and adrenergic blockers.[87][88][89]
Burnstock's proposal was met with criticism, since ATP is an ubiquitous intracellular molecular energy source[90] so it seemed counter-intuitive that cells might also actively release this vital molecule as a neurotransmitter. After years of prolonged scepticism, however, the concept of purinergic signalling was gradually accepted by the scientific community.[1]
Today, purinergic signalling is no longer considered to be confined to neurotransmission, but is regarded as a general intercellular communication system of many, if not all, tissues.[1]
See also
[edit]References
[edit]- ^ a b c d Praetorius HA, Leipziger J (1 March 2010). "Intrarenal purinergic signaling in the control of renal tubular transport". Annual Review of Physiology. 72 (1): 377–93. doi:10.1146/annurev-physiol-021909-135825. PMID 20148681.
- ^ Burnstock, Geoffrey (March 2012). "Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future". BioEssays. 34 (3): 218–225. doi:10.1002/bies.201100130. ISSN 0265-9247.
- ^ Burnstock, Geoffrey (January 2018). "Purine and purinergic receptors". Brain and Neuroscience Advances. 2: 239821281881749. doi:10.1177/2398212818817494. ISSN 2398-2128. PMC 7058212. PMID 32166165.
- ^ Volonté, Cinzia; D’Ambrosi, Nadia (2009). "Membrane compartments and purinergic signalling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters: The purinome". FEBS Journal. 276 (2): 318–329. doi:10.1111/j.1742-4658.2008.06793.x. PMID 19076212.
- ^ Tanaka K, Gilroy S, Jones AM, Stacey G (October 2010). "Extracellular ATP signaling in plants". Trends in Cell Biology. 20 (10): 601–8. doi:10.1016/j.tcb.2010.07.005. PMC 4864069. PMID 20817461.
- ^ Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H (January 2009). "Purinergic signalling in the nervous system: an overview". Trends in Neurosciences. 32 (1): 19–29. doi:10.1016/j.tins.2008.10.001. PMID 19008000. S2CID 7653609.
- ^ Burnstock G, Verkhratsky A (April 2009). "Evolutionary origins of the purinergic signalling system". Acta Physiologica. 195 (4): 415–47. doi:10.1111/j.1748-1716.2009.01957.x. PMID 19222398. S2CID 12644331.
- ^ Roux SJ, Steinebrunner I (November 2007). "Extracellular ATP: an unexpected role as a signaler in plants". Trends in Plant Science. 12 (11): 522–7. doi:10.1016/j.tplants.2007.09.003. PMID 17928260.
- ^ Cao Y, Tanaka K, Nguyen CT, Stacey G (August 2014). "Extracellular ATP is a central signaling molecule in plant stress responses". Current Opinion in Plant Biology. 20: 82–7. doi:10.1016/j.pbi.2014.04.009. PMID 24865948.
- ^ Fountain SJ (December 2013). "Primitive ATP-activated P2X receptors: discovery, function and pharmacology". Frontiers in Cellular Neuroscience. 7: 247. doi:10.3389/fncel.2013.00247. PMC 3853471. PMID 24367292.
- ^ Sperlagh B, Csolle C, Ando RD, Goloncser F, Kittel A, Baranyi M (December 2012). "The role of purinergic signaling in depressive disorders". Neuropsychopharmacologia Hungarica. 14 (4): 231–8. PMID 23269209.
- ^ Corriden R, Insel PA (January 2010). "Basal release of ATP: an autocrine-paracrine mechanism for cell regulation". Science Signaling. 3 (104): re1. doi:10.1126/scisignal.3104re1. PMC 3085344. PMID 20068232.
Cells release other nucleotides [for example, uridine triphosphate (UTP) and related molecules such as uridine diphosphate (UDP) sugars] that have actions akin to those of ATP
- ^ a b c Eltzschig HK, Sitkovsky MV, Robson SC (December 2012). "Purinergic signaling during inflammation". The New England Journal of Medicine. 367 (24): 2322–33. doi:10.1056/NEJMra1205750. PMC 3675791. PMID 23234515.
- ^ a b c d Dos Santos-Rodrigues A, Grañé-Boladeras N, Bicket A, Coe IR (July 2014). "Nucleoside transporters in the purinome". Neurochemistry International. 73: 229–37. doi:10.1016/j.neuint.2014.03.014. PMID 24704797. S2CID 24292160.
- ^ Kukulski F, Lévesque SA, Sévigny J (2011). "Impact of ectoenzymes on p2 and p1 receptor signaling". Pharmacology of Purine and Pyrimidine Receptors. Advances in Pharmacology. Vol. 61. pp. 263–99. doi:10.1016/B978-0-12-385526-8.00009-6. ISBN 9780123855268. PMID 21586362.
- ^ Zimmermann H, Zebisch M, Sträter N (September 2012). "Cellular function and molecular structure of ecto-nucleotidases". Purinergic Signalling. 8 (3): 437–502. doi:10.1007/s11302-012-9309-4. PMC 3360096. PMID 22555564.
- ^ Makarenkova HP, Shestopalov VI (2014). "The role of pannexin hemichannels in inflammation and regeneration". Frontiers in Physiology. 5: 63. doi:10.3389/fphys.2014.00063. PMC 3933922. PMID 24616702.
- ^ Adamson SE, Leitinger N (April 2014). "The role of pannexin1 in the induction and resolution of inflammation". FEBS Letters. 588 (8): 1416–22. doi:10.1016/j.febslet.2014.03.009. PMC 4060616. PMID 24642372.
- ^ McIntosh VJ, Lasley RD (March 2012). "Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant?". Journal of Cardiovascular Pharmacology and Therapeutics. 17 (1): 21–33. doi:10.1177/1074248410396877. PMID 21335481. S2CID 544367.
- ^ Mustafa SJ, Morrison RR, Teng B, Pelleg A (2009). "Adenosine Receptors and the Heart: Role in Regulation of Coronary Blood Flow and Cardiac Electrophysiology". Adenosine Receptors in Health and Disease. Handbook of Experimental Pharmacology. Vol. 193. pp. 161–88. doi:10.1007/978-3-540-89615-9_6. ISBN 978-3-540-89614-2. PMC 2913612. PMID 19639282.
- ^ a b Colgan SP, Eltzschig HK (17 March 2012). "Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery". Annual Review of Physiology. 74 (1): 153–75. doi:10.1146/annurev-physiol-020911-153230. PMC 3882030. PMID 21942704.
- ^ Lohman AW, Billaud M, Isakson BE (August 2012). "Mechanisms of ATP release and signalling in the blood vessel wall". Cardiovascular Research. 95 (3): 269–80. doi:10.1093/cvr/cvs187. PMC 3400358. PMID 22678409.
- ^ Dahl G, Muller KJ (April 2014). "Innexin and pannexin channels and their signaling". FEBS Letters. 588 (8): 1396–402. doi:10.1016/j.febslet.2014.03.007. PMID 24632288. S2CID 45630385.
- ^ Storey RF (August 2011). "New P2Y₁₂ inhibitors". Heart. 97 (15): 1262–7. doi:10.1136/hrt.2009.184242. PMID 21742618. S2CID 5140764.
- ^ Barn K, Steinhubl SR (September 2012). "A brief review of the past and future of platelet P2Y12 antagonist". Coronary Artery Disease. 23 (6): 368–74. doi:10.1097/MCA.0b013e3283564930. PMID 22735090. S2CID 2870694.
- ^ Oliveira AG, Marques PE, Amaral SS, Quintão JL, Cogliati B, Dagli ML, Rogiers V, Vanhaecke T, Vinken M, Menezes GB (March 2013). "Purinergic signalling during sterile liver injury". Liver International. 33 (3): 353–61. doi:10.1111/liv.12109. PMID 23402607.
- ^ Wood JD (December 2006). "The enteric purinergic P2Y1 receptor". Current Opinion in Pharmacology. 6 (6): 564–70. doi:10.1016/j.coph.2006.06.006. PMID 16934527.
- ^ Stojilkovic SS, Koshimizu T (July 2001). "Signaling by extracellular nucleotides in anterior pituitary cells". Trends in Endocrinology and Metabolism. 12 (5): 218–25. doi:10.1016/S1043-2760(01)00387-3. PMID 11397647. S2CID 21874995.
- ^ Junger WG (March 2011). "Immune cell regulation by autocrine purinergic signalling". Nature Reviews. Immunology. 11 (3): 201–12. doi:10.1038/nri2938. PMC 4209705. PMID 21331080.
- ^ "Final Report Summary - ATPBONE (Fighting osteoporosis by blocking nucleotides: purinergic signalling in bone formation and homeostasis)". CORDIS. Retrieved 4 September 2013.
- ^ Jacob F, Pérez Novo C, Bachert C, Van Crombruggen K (September 2013). "Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses". Purinergic Signalling. 9 (3): 285–306. doi:10.1007/s11302-013-9357-4. PMC 3757148. PMID 23404828.
- ^ Trautmann A (February 2009). "Extracellular ATP in the immune system: more than just a "danger signal"". Science Signaling. 2 (56): pe6. doi:10.1126/scisignal.256pe6. PMID 19193605. S2CID 42651032.
- ^ Barletta KE, Ley K, Mehrad B (April 2012). "Regulation of neutrophil function by adenosine". Arteriosclerosis, Thrombosis, and Vascular Biology. 32 (4): 856–64. doi:10.1161/atvbaha.111.226845. PMC 3353547. PMID 22423037.
- ^ Eltzschig HK, Macmanus CF, Colgan SP (April 2008). "Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface". Trends in Cardiovascular Medicine. 18 (3): 103–7. doi:10.1016/j.tcm.2008.01.006. PMC 2711033. PMID 18436149.
- ^ North RA, Verkhratsky A (August 2006). "Purinergic transmission in the central nervous system". Pflügers Archiv. 452 (5): 479–85. doi:10.1007/s00424-006-0060-y. PMID 16688467. S2CID 25006319.
- ^ Ransohoff RM, Perry VH (April 2009). "Microglial physiology: unique stimuli, specialized responses". Annual Review of Immunology. 27 (1): 119–45. doi:10.1146/annurev.immunol.021908.132528. PMID 19302036.
- ^ Fields RD, Burnstock G (June 2006). "Purinergic signalling in neuron-glia interactions". Nature Reviews. Neuroscience. 7 (6): 423–36. doi:10.1038/nrn1928. PMC 2062484. PMID 16715052.
- ^ Cserép C, Pósfai B, Lénárt N, Fekete R, László ZI, Lele Z, et al. (January 2020). "Microglia monitor and protect neuronal function through specialized somatic purinergic junctions". Science. 367 (6477): 528–537. Bibcode:2020Sci...367..528C. doi:10.1126/science.aax6752. PMID 31831638. S2CID 209343260.
- ^ Csaba, Cserep; Anett, Schwarcz D (2022). "Microglial control of neuronal development via somatic purinergic junctions". Cell Reports. doi:10.1016/j.celrep.2022.111369. PMID 36130488.
- ^ Lohr C, Grosche A, Reichenbach A, Hirnet D (October 2014). "Purinergic neuron-glia interactions in sensory systems". Pflügers Archiv. 466 (10): 1859–72. doi:10.1007/s00424-014-1510-6. PMID 24705940. S2CID 18952066.
- ^ a b Del Puerto A, Wandosell F, Garrido JJ (October 2013). "Neuronal and glial purinergic receptors functions in neuron development and brain disease". Frontiers in Cellular Neuroscience. 7: 197. doi:10.3389/fncel.2013.00197. PMC 3808753. PMID 24191147.
- ^ Peter, Berki; Csaba, Cserep; Zsuzsanna, Környei (2024). "Microglia contribute to neuronal synchrony despite endogenous ATP-related phenotypic transformation in acute mouse brain slices". Nature Communications. doi:10.1038/s41467-024-49773-1. PMC 11208608. PMID 38926390.
- ^ Arulkumaran N, Turner CM, Sixma ML, Singer M, Unwin R, Tam FW (1 January 2013). "Purinergic signaling in inflammatory renal disease". Frontiers in Physiology. 4: 194. doi:10.3389/fphys.2013.00194. PMC 3725473. PMID 23908631.
Extracellular adenosine contributes to the regulation of GFR. Renal interstitial adenosine is mainly derived from dephosphorylation of released ATP, AMP, or cAMP by the enzyme ecto-5′-nucleotidase (CD73) (Le Hir and Kaissling, 1993). This enzyme catalyzes the dephosphorylation of 5′-AMP or 5′-IMP to adenosine or inosine, respectively, and is located primarily on the external membranes and mitochondria of proximal tubule cells, but not in distal tubule or collecting duct cells (Miller et al., 1978). ATP consumed in active transport by the macula densa also contributes to the formation of adenosine by 5- nucleotidase (Thomson et al., 2000). Extracellular adenosine activates A1 receptors on vascular afferent arteriolar smooth muscle cells, resulting in vasoconstriction and a reduction in GFR (Schnermann et al., 1990).
- ^ Ren Y, Garvin JL, Liu R, Carretero OA (October 2004). "Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback". Kidney International. 66 (4): 1479–85. doi:10.1111/j.1523-1755.2004.00911.x. PMID 15458441.
- ^ a b Lazarowski ER, Boucher RC (June 2009). "Purinergic receptors in airway epithelia". Current Opinion in Pharmacology. 9 (3): 262–7. doi:10.1016/j.coph.2009.02.004. PMC 2692813. PMID 19285919.
- ^ Housley GD (October 2011). "Recent insights into the regulation of breathing". Autonomic Neuroscience. 164 (1–2): 3–5. doi:10.1016/j.autneu.2011.08.002. PMID 21852203. S2CID 30097466.
- ^ a b Rumney RM, Wang N, Agrawal A, Gartland A (2012). "Purinergic signalling in bone". Frontiers in Endocrinology. 3: 116. doi:10.3389/fendo.2012.00116. PMC 3446723. PMID 23049524.
- ^ Mediero A, Cronstein BN (June 2013). "Adenosine and bone metabolism". Trends in Endocrinology and Metabolism. 24 (6): 290–300. doi:10.1016/j.tem.2013.02.001. PMC 3669669. PMID 23499155.
- ^ Wilson CN (October 2008). "Adenosine receptors and asthma in humans". British Journal of Pharmacology. 155 (4): 475–86. doi:10.1038/bjp.2008.361. PMC 2579661. PMID 18852693.
- ^ Jørgensen NR, Adinolfi E, Orriss I, Schwarz P (1 January 2013). "Purinergic signaling in bone". Journal of Osteoporosis. 2013: 673684. doi:10.1155/2013/673684. PMC 3671543. PMID 23762774.
- ^ Di Virgilio F (November 2012). "Purines, purinergic receptors, and cancer". Cancer Research (Editorial). 72 (21): 5441–7. doi:10.1158/0008-5472.CAN-12-1600. PMID 23090120.
- ^ Antonioli L, Blandizzi C, Pacher P, Haskó G (December 2013). "Immunity, inflammation and cancer: a leading role for adenosine". Nature Reviews. Cancer. 13 (12): 842–57. doi:10.1038/nrc3613. PMID 24226193. S2CID 13224098.
- ^ Reiss AB, Cronstein BN (April 2012). "Regulation of foam cells by adenosine". Arteriosclerosis, Thrombosis, and Vascular Biology. 32 (4): 879–86. doi:10.1161/atvbaha.111.226878. PMC 3306592. PMID 22423040.
- ^ Mortaz E, Folkerts G, Nijkamp FP, Henricks PA (July 2010). "ATP and the pathogenesis of COPD". European Journal of Pharmacology. 638 (1–3): 1–4. doi:10.1016/j.ejphar.2010.04.019. PMID 20423711.
- ^ Esther CR, Alexis NE, Picher M (2011). "Regulation of Airway Nucleotides in Chronic Lung Diseases". Purinergic Regulation of Respiratory Diseases. Subcellular Biochemistry. Vol. 55. pp. 75–93. doi:10.1007/978-94-007-1217-1_4. ISBN 978-94-007-1216-4. PMID 21560045.
- ^ Phatarpekar PV, Wen J, Xia Y (November 2010). "Role of adenosine signaling in penile erection and erectile disorders". The Journal of Sexual Medicine. 7 (11): 3553–64. doi:10.1111/j.1743-6109.2009.01555.x. PMC 2906687. PMID 19889148.
- ^ Wen J, Xia Y (April 2012). "Adenosine signaling: good or bad in erectile function?". Arteriosclerosis, Thrombosis, and Vascular Biology. 32 (4): 845–50. doi:10.1161/atvbaha.111.226803. PMID 22423035.
- ^ a b Lu D, Insel PA (May 2014). "Cellular mechanisms of tissue fibrosis. 6. Purinergic signaling and response in fibroblasts and tissue fibrosis". American Journal of Physiology. Cell Physiology. 306 (9): C779-88. doi:10.1152/ajpcell.00381.2013. PMC 4010809. PMID 24352335.
- ^ Karmouty-Quintana H, Xia Y, Blackburn MR (February 2013). "Adenosine signaling during acute and chronic disease states". Journal of Molecular Medicine. 91 (2): 173–81. doi:10.1007/s00109-013-0997-1. PMC 3606047. PMID 23340998.
- ^ Blazar BR, Murphy WJ, Abedi M (May 2012). "Advances in graft-versus-host disease biology and therapy". Nature Reviews. Immunology. 12 (6): 443–58. doi:10.1038/nri3212. PMC 3552454. PMID 22576252.
- ^ Doll J, Zeitler E, Becker R (July 2013). "Generic clopidogrel: time to substitute?". JAMA. 310 (2): 145–6. doi:10.1001/jama.2013.7155. PMID 23839745.
- ^ Berman BM, Langevin HM, Witt CM, Dubner R (July 2010). "Acupuncture for chronic low back pain". The New England Journal of Medicine. 363 (5): 454–61. doi:10.1056/NEJMct0806114. PMID 20818865.
Acupuncture also has effects on local tissues, including mechanical stimulation of connective tissue, release of adenosine at the site of needle stimulation, and increases in local blood flow
- ^ Sawynok J (2013). "Adenosine and Pain". In Masino S, Boison D (eds.). Adenosine. New York, NY: Springer. p. 352. doi:10.1007/978-1-4614-3903-5_17. ISBN 978-1-4614-3903-5.
in an elegant series of experiments, adenosine has been implicated as a mediator of acupuncture analgesia
{{cite book}}:|work=ignored (help) - ^ Gorski, David H. (2014). "Integrative oncology: really the best of both worlds?". Nature Reviews Cancer. 14 (10): 692–700. doi:10.1038/nrc3822. ISSN 1474-175X. PMID 25230880. S2CID 33539406.
- ^ Yang ES, Li PW, Nilius B, Li G (November 2011). "Ancient Chinese medicine and mechanistic evidence of acupuncture physiology". Pflügers Archiv. 462 (5): 645–53. doi:10.1007/s00424-011-1017-3. PMC 3192271. PMID 21870056.
Anti-nociceptive effect of acupuncture requires A1 receptors
- ^ Zylka MJ (April 2011). "Pain-relieving prospects for adenosine receptors and ectonucleotidases". Trends in Molecular Medicine. 17 (4): 188–96. doi:10.1016/j.molmed.2010.12.006. PMC 3078941. PMID 21236731.
Antinociceptive effects of acupuncture require A1R activation
- ^ Langevin HM (2014). "Acupuncture, connective tissue, and peripheral sensory modulation". Critical Reviews in Eukaryotic Gene Expression. 24 (3): 249–53. doi:10.1615/CritRevEukaryotGeneExpr.2014008284. PMID 25072149.
- ^ Zhang R, Lao L, Ren K, Berman BM (February 2014). "Mechanisms of acupuncture-electroacupuncture on persistent pain". Anesthesiology. 120 (2): 482–503. doi:10.1097/ALN.0000000000000101. PMC 3947586. PMID 24322588.
- ^ Chan ES, Cronstein BN (2002). "Molecular action of methotrexate in inflammatory diseases". Arthritis Research. 4 (4): 266–73. doi:10.1186/ar419. PMC 128935. PMID 12106498.
- ^ Topol EJ, Schork NJ (January 2011). "Catapulting clopidogrel pharmacogenomics forward". Nature Medicine. 17 (1): 40–1. doi:10.1038/nm0111-40. PMID 21217678. S2CID 32083067.
- ^ Barnes PJ (October 2013). "Theophylline". American Journal of Respiratory and Critical Care Medicine. 188 (8): 901–6. doi:10.1164/rccm.201302-0388PP. PMID 23672674.
- ^ a b Liang S, Xu C, Li G, Gao Y (December 2010). "P2X receptors and modulation of pain transmission: focus on effects of drugs and compounds used in traditional Chinese medicine". Neurochemistry International. 57 (7): 705–12. doi:10.1016/j.neuint.2010.09.004. PMID 20863868. S2CID 21358206.
- ^ Burnstock G (March 2006). "Pathophysiology and therapeutic potential of purinergic signaling". Pharmacological Reviews. 58 (1): 58–86. CiteSeerX 10.1.1.623.4370. doi:10.1124/pr.58.1.5. PMID 16507883. S2CID 12337865.
Tetramethylpyrazine, a traditional Chinese medicine used as an analgesic for dysmenorrhea, was shown to block P2X3 receptor signaling
- ^ Burnstock G (June 2006). "Purinergic P2 receptors as targets for novel analgesics". Pharmacology & Therapeutics. 110 (3): 433–54. doi:10.1016/j.pharmthera.2005.08.013. PMID 16226312.
- ^ Burnstock G, Knight GE, Greig AV (March 2012). "Purinergic signaling in healthy and diseased skin". The Journal of Investigative Dermatology. 132 (3 Pt 1): 526–46. doi:10.1038/jid.2011.344. PMID 22158558.
- ^ Zhou YX, Zhang H, Peng C (July 2014). "Puerarin: a review of pharmacological effects". Phytotherapy Research. 28 (7): 961–75. doi:10.1002/ptr.5083. PMID 24339367. S2CID 40855672.
- ^ Jiang LH, Baldwin JM, Roger S, Baldwin SA (2013). "Insights into the Molecular Mechanisms Underlying Mammalian P2X7 Receptor Functions and Contributions in Diseases, Revealed by Structural Modeling and Single Nucleotide Polymorphisms". Frontiers in Pharmacology. 4: 55. doi:10.3389/fphar.2013.00055. PMC 3646254. PMID 23675347.
Natural compounds isolated from plants used in traditional medicines have also been shown to selectively inhibit the P2X7Rs
- ^ Adinolfi E (December 2013). "New intriguing roles of ATP and its receptors in promoting tumor metastasis : presented by Maria P. Abbracchio". Purinergic Signalling. 9 (4): 487–90. doi:10.1007/s11302-013-9401-4. PMC 3889383. PMID 24258487.
The study from Jelassi and colleagues further support these findings showing the efficacy of emodin, a Chinese traditional medicine compound, in reducing P2X7 mediated malignant progression.
- ^ Burnstock G, Di Virgilio F (December 2013). "Purinergic signalling and cancer". Purinergic Signalling. 9 (4): 491–540. doi:10.1007/s11302-013-9372-5. PMC 3889385. PMID 23797685.
Chrysophanol, a member of the anthraquinone family that is one of the components of a Chinese herb including rhubarb recommended for the treatment of cancer, induces necrosis of J5 human liver cancer cells via reduction in ATP levels
- ^ Chen JF, Eltzschig HK, Fredholm BB (April 2013). "Adenosine receptors as drug targets--what are the challenges?". Nature Reviews. Drug Discovery. 12 (4): 265–86. doi:10.1038/nrd3955. PMC 3930074. PMID 23535933.
- ^ Palani G, Ananthasubramaniam K (2013). "Regadenoson: review of its established role in myocardial perfusion imaging and emerging applications". Cardiology in Review. 21 (1): 42–8. doi:10.1097/CRD.0b013e3182613db6. PMID 22643345. S2CID 9183656.
- ^ Cerqueira MD (July 2004). "The future of pharmacologic stress: selective A2A adenosine receptor agonists". The American Journal of Cardiology. 94 (2A): 33D–40D, discussion 40D-42D. doi:10.1016/j.amjcard.2004.04.017. PMID 15261132.
- ^ Lau OC, Samarawickrama C, Skalicky SE (January 2014). "P2Y2 receptor agonists for the treatment of dry eye disease: a review". Clinical Ophthalmology. 8: 327–34. doi:10.2147/OPTH.S39699. PMC 3915022. PMID 24511227.
- ^ Chen W, Wang H, Wei H, Gu S, Wei H (January 2013). "Istradefylline, an adenosine A₂A receptor antagonist, for patients with Parkinson's Disease: a meta-analysis". Journal of the Neurological Sciences. 324 (1–2): 21–8. doi:10.1016/j.jns.2012.08.030. PMID 23085003. S2CID 34848760.
- ^ Drury AN, Szent-Györgyi A (November 1929). "The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart". The Journal of Physiology. 68 (3): 213–37. doi:10.1113/jphysiol.1929.sp002608. PMC 1402863. PMID 16994064.
- ^ Campbell G (April 1987). "Cotransmission". Annual Review of Pharmacology and Toxicology. 27 (1): 51–70. doi:10.1146/annurev.pa.27.040187.000411. PMID 2883929.
- ^ Martinson J, Muren A (1963). "Excitatory and inhibitory effects if vagus stimulation on gastric motility in the cat". Acta Physiol. Scand. 57 (4): 309–316. doi:10.1111/j.1748-1716.1963.tb02594.x.
- ^ Burnstock G, Campbell G, Bennett M, Holman ME (November 1963). "Inhibition of the Smooth Muscle on the Taenia Coli". Nature. 200 (4906): 581–2. Bibcode:1963Natur.200..581B. doi:10.1038/200581a0. PMID 14082235. S2CID 4277023.
- ^ Burnstock G (September 1972). "Purinergic nerves". Pharmacological Reviews. 24 (3): 509–81. PMID 4404211.
- ^ Lipmann F (1941). "Metabolic Generation and Utilization of Phosphate Bond Energy". In Nord FF, Werkman CH (eds.). Advances in Enzymology and Related Areas of Molecular Biology. Vol. 1. pp. 99–162. doi:10.1002/9780470122464.ch4. ISBN 9780470122464. S2CID 94733045.
