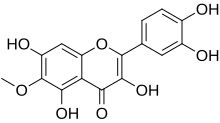
Patuletin
Appearance
 | |
| Names | |
|---|---|
| IUPAC name
3,3′,4′,5,7-Pentahydroxy-6-methoxyflavone
| |
| Systematic IUPAC name
2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-6-methoxy-4H-1-benzopyran-4-one | |
| Other names
6-Methoxyquercetin
Quercetagetin 6-methyl ether 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-6-methoxy-4-benzopyrone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.529 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12O8 | |
| Molar mass | 332.264 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Patuletin is an O-methylated flavonol. It can be found in the genus Eriocaulon.[1]
Glycosides
[edit]Patuletin glycosides can be found in Ipomopsis aggregata.[2]
Patuletin-3-O-rutinoside can be isolated from the aerial parts of Echinacea angustifolia.[3]
Patuletin acetylrhamnosides can be isolated from Kalanchoe brasiliensis.[4]
References
[edit]- ^ Bate-Smith, E. C.; Harborne, J. B. (1969). "Quercetagetin and patuletin in Eriocaulon". Phytochemistry. 8 (6): 1035. Bibcode:1969PChem...8.1035B. doi:10.1016/S0031-9422(00)86351-7.
- ^ Smith, D. M.; Glennie, C. W.; Harborne, J. B. (1971). "Identification of eupalitin, eupatolitin and patuletin glycosides in Ipomopsis aggregata". Phytochemistry. 10 (12): 3115. Bibcode:1971PChem..10.3115S. doi:10.1016/S0031-9422(00)97361-8.
- ^ Lin, L.; Qiu, S.; Lindenmaier, M.; He, X.; Featherstone, T.; Cordell, G. A. (2002). "Patuletin-3-O-Rutinoside from the Aerial Parts of Echinacea angustifolia". Pharmaceutical Biology. 40 (2): 92. doi:10.1076/phbi.40.2.92.5839. S2CID 84855629.
- ^ Costa, S. S.; Jossang, A.; Bodo, B.; Souza, M. L. M.; Moraes, V. L. G. (1994). "Patuletin Acetylrhamnosides from Kalanchoe brasiliensis as Inhibitors of Human Lymphocyte Proliferative Activity". Journal of Natural Products. 57 (11): 1503–1510. doi:10.1021/np50113a005. PMID 7853000.
