Mono-N-protected amino acids
Mono-N-protected amino acid (MPAA) is a bifunctional ligand that plays a key role in C–H functionalizations by accelerating the reaction rate and imparting specified chirality into the product.[1] Amino acids are ideal building blocks for chiral ligand synthesis due to the cost, accessibility, large variety, solubility, and inherent chirality.[2] Naturally occurring amino acids are transformed into chiral MPAA ligands that, upon coordination to metal complexes, allow reactions to occur that are otherwise energetically unfavorable. Great strides in the development of MPAA ligands over the past two decades have led to the integral role that enantioselective catalysis now plays in complex organic synthesis.[3]
History and development
[edit]
In the past century, there has been much research into the development of effective chiral catalysts due to its great potential in organic synthesis.[4] In the 1960s, cyclometalation reactions including C(sp2)–H and C(sp3)–H cleavage were pioneered by Kleiman, Dubeck, Cope, and Siekman.[5][6] A decade later, Shaw discovered that inorganic acetate salts promoted otherwise difficult cyclopalladations.[7] To build off of this work, Sokolov focused on developing chiral, enantioenriched metallacyles and proposed the concerted metal-deprotonation (CMD) mechanism.[8]
Despite this foundation of discoveries, enantioselective catalysis for C–H functionalization continued to lack in efficiency oand selectivity for desired chiral product formation.[3] In 2008, Jin-Quan Yu reported the first MPAA ligands, showcasing their use in enantioselective activation of C(sp2)–H and C(sp3)–H bonds.[9] Initial synthesis occurred by reacting the nucleophilic amino acid in base with a highly electrophilic acyl chloride resulting in one new amide bond formation. Upon addition of acyl chloride, most resulting groups off of the nitrogen were common protecting groups used in organic synthesis, hence mono-N-protected. Taking advantage of the weak coordination of amides and carboxylates with Pd-complexes, this enantioselective catalysis requires the MPAA ligand to allow the reaction to proceed and determine the product chirality, minimizing side reactions that may occur without the ligand.[1] Since the initial discovery, Yu has continued to pioneer the field by expanding the substrate scope, increasing functional group tolerance, and developing ligand variations.
Mechanism
[edit]After iterative computational and experimental studies, the internal amidate mechanism was proposed in collaboration of Wu, Yu, and Houk.[10][11] In the proposed mechanism, the trimeric Pd-precatalyst converts to the mono-Pd complex with coordination to solvent and the bidentate MPAA ligand. Mass spectrometry results reveal this active catalyst which forms favorably with the stabilizing dianionic MPAA ligand as computations suggest.

The key intermediate step of these cyclometalation reactions involves the metal-mediated cleavage of the C–H bond and simultaneous formation of the metal–C bond of the substrate. Upon addition of substrate, the N-acyl motif acts as an internal proton acceptor in the concerted metal-deprotonation (CMD) of the transition state for this inner-sphere process. According to this model, the rate and selectivity of the C–H functionalization are impacted by the basicity of the MPAA ligand. The resulting experimental data of steric and electronic alteration of the MPAA ligands align with this model.[12] In 2023, the first experimental observations to support the proposed mechanism were reported, which were previously unattainable due to the lack of well-defined isolated palladium-MPAA complexes.[13]
Ligand variations
[edit]Since the initial MPAA ligand report, many variations of bifunctional ligands derived from amino acids have been developed. Bidentate MPAQ (mono-protected amino quinoline) ligands were introduced in the application of β-methylene C–H bonds in aliphatic amides.[14] The highly successful MPAO (mono-protected amino oxazoline) ligand allowed for C(sp3)–H functionalization via arylation of α-methyls, borylation of cyclobutyl carboxylic amides, and boronic cross coupling of alkyl amines.[15][16][17][18] MPAAM (mono-protected aminoalkyl amine) ligands were used in enantioselective C(sp3)–H arylations of free aliphatic acids without the need for exogenous directing groups.[19] Variations of the MPAAThio (mono-protected aminoalkyl thioether) ligands have been use in olefination of free carboxylic acids and arylation, carbonylation, and olefination of free aliphatic amines.[20][21]
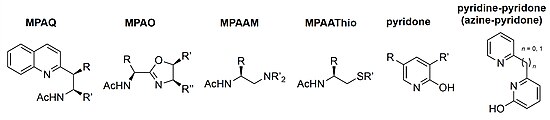
Expanding the reaction substrate scope to non-directed C(sp2)–H bonds, pyridone ligands were developed to functionalize arenes and heteroarenes which proved to be particularly useful in late-state derivatization of bioactive compounds such as estrone, caffeine, and camptothecin.[22][23] Many analogues of the pyridine-pyridone (azine-pyridone) ligands were developed and used in the C(sp2)–H hydroxylation of (hetero)arenes and the dehydrogenation of methylene C(sp3)–H bonds on alkyl free acids.[24][25]
Applications in total synthesis
[edit]
The development of MPAA ligands enabled and improved the synthesis of many complex natural products. Examples include Arnottin 1,[26] Aspercylide B,[27] Berkelic Acid,[28] Boletopsin 11,[29] Danshenspiroketallactone,[29] Delavatine A,[30] Herbindole B/cis-Trikentrin A,[31] Hongoquercin A,[32] Incarviatone A,[33] Indoxamycin,[34] Kedarcidin/Neocarzinostatin,[35] Kinamycin[36]
, Lithospermic Acid,[37] M1 PAMs,[38] and VS-548.[39] In the formation of indoxamycin cores, MPAA ligand assisted C–H functionalization introduces high complexity via intramolecular ortho olefination.[34]

The use of C–H functionalization in the synthesis of lithospheric acid exemplifies the site- and stereoselective capabilities of using MPAA ligands in these reactions. As the penultimate step, the intermolecular C–H olefination introduces almost double the complexity into the compound enabling the highly convergent synthesis.[37]
References
[edit]- ^ a b Engle, Keary M. (2016). "The mechanism of palladium(II)-mediated C–H cleavage with mono-N-protected amino acid (MPAA) ligands: origins of rate acceleration". Pure and Applied Chemistry. 88 (1–2): 119–138. doi:10.1515/pac-2015-0902. ISSN 1365-3075.
- ^ Ullmann's Encyclopedia of Industrial Chemistry (1st ed.). Wiley. 2000-06-15. doi:10.1002/14356007.a02_057.pub2. ISBN 978-3-527-30385-4.
- ^ a b Shao, Qian; Wu, Kevin; Zhuang, Zhe; Qian, Shaoqun; Yu, Jin-Quan (2020-04-21). "From Pd(OAc) 2 to Chiral Catalysts: The Discovery and Development of Bifunctional Mono-N-Protected Amino Acid Ligands for Diverse C–H Functionalization Reactions". Accounts of Chemical Research. 53 (4): 833–851. doi:10.1021/acs.accounts.9b00621. ISSN 0001-4842. PMC 7738004. PMID 32227915.
- ^ Arndtsen, Bruce A.; Bergman, Robert G.; Mobley, T. Andrew; Peterson, Thomas H. (1995-03-01). "Selective Intermolecular Carbon-Hydrogen Bond Activation by Synthetic Metal Complexes in Homogeneous Solution". Accounts of Chemical Research. 28 (3): 154–162. doi:10.1021/ar00051a009. ISSN 0001-4842.
- ^ Kleiman, Joseph P.; Dubeck, Michael. (May 1963). "The Preparation of Cyclopentadienyl [ o -(Phenylazo)Phenyl]Nickel". Journal of the American Chemical Society. 85 (10): 1544–1545. doi:10.1021/ja00893a040. ISSN 0002-7863.
- ^ Cope, Arthur C.; Siekman, Robert W. (July 1965). "Formation of Covalent Bonds from Platinum or Palladium to Carbon by Direct Substitution". Journal of the American Chemical Society. 87 (14): 3272–3273. doi:10.1021/ja01092a063. ISSN 0002-7863.
- ^ Gaunt, John C.; Shaw, Bernard L. (1975-12-23). "Transition metalcarbon bonds: XL. Palladium(II) complexes of [(dimethylamino)methyl]-ferrocene". Journal of Organometallic Chemistry. 102 (4): 511–516. doi:10.1016/S0022-328X(00)89395-X. ISSN 0022-328X.
- ^ Sokolov, V. I.; Troitskaya, L. L.; Reutov, O. A. (1979-12-18). "Asymmetric cyclopalladation of dimethylaminomethylferrocene". Journal of Organometallic Chemistry. 182 (4): 537–546. doi:10.1016/S0022-328X(00)83942-X. ISSN 0022-328X.
- ^ Shi, Bing-Feng; Maugel, Nathan; Zhang, Yang-Hui; Yu, Jin-Quan (2008-06-16). "PdII-Catalyzed Enantioselective Activation of C(sp2)H and C(sp3)H Bonds Using Monoprotected Amino Acids as Chiral Ligands". Angewandte Chemie International Edition. 47 (26): 4882–4886. doi:10.1002/anie.200801030. PMID 18484582.
- ^ Musaev, Djamaladdin G.; Kaledin, Alexey; Shi, Bing-Feng; Yu, Jin-Quan (2012-01-25). "Key Mechanistic Features of Enantioselective C–H Bond Activation Reactions Catalyzed by [(Chiral Mono- N -Protected Amino Acid)–Pd(II)] Complexes". Journal of the American Chemical Society. 134 (3): 1690–1698. doi:10.1021/ja208661v. ISSN 0002-7863. PMID 22148424.
- ^ Cheng, Gui-Juan; Yang, Yun-Fang; Liu, Peng; Chen, Ping; Sun, Tian-Yu; Li, Gang; Zhang, Xinhao; Houk, K. N.; Yu, Jin-Quan; Wu, Yun-Dong (2014-01-22). "Role of N -Acyl Amino Acid Ligands in Pd(II)-Catalyzed Remote C–H Activation of Tethered Arenes". Journal of the American Chemical Society. 136 (3): 894–897. doi:10.1021/ja411683n. ISSN 0002-7863. PMID 24410499.
- ^ Park, Yoonsu; Niemeyer, Zachary L.; Yu, Jin-Quan; Sigman, Matthew S. (2018-01-22). "Quantifying Structural Effects of Amino Acid Ligands in Pd(II)-Catalyzed Enantioselective C–H Functionalization Reactions". Organometallics. 37 (2): 203–210. doi:10.1021/acs.organomet.7b00751. ISSN 0276-7333.
- ^ Fernández-Moyano, Sara; Salamanca, Vanesa; Albéniz, Ana C. (2023-05-26). "Palladium mono-N-protected amino acid complexes: experimental validation of the ligand cooperation model in C–H activation". Chemical Science. 14 (24): 6688–6694. doi:10.1039/D3SC02076B. ISSN 2041-6539. PMC 10284104. PMID 37350841. S2CID 258943376.
- ^ Chen, Gang; Gong, Wei; Zhuang, Zhe; Andrä, Michal S.; Chen, Yan-Qiao; Hong, Xin; Yang, Yun-Fang; Liu, Tao; Houk, K. N.; Yu, Jin-Quan (2016-09-02). "Ligand-accelerated enantioselective methylene C(sp 3 )–H bond activation". Science. 353 (6303): 1023–1027. doi:10.1126/science.aaf4434. ISSN 0036-8075. PMC 5516954. PMID 27701111.
- ^ Wu, Qing-Feng; Shen, Peng-Xiang; He, Jian; Wang, Xiao-Bing; Zhang, Forrest; Shao, Qian; Zhu, Ru-Yi; Mapelli, Claudio; Qiao, Jennifer X.; Poss, Michael A.; Yu, Jin-Quan (2017-02-03). "Formation of α-chiral centers by asymmetric β-C(sp 3 )–H arylation, alkenylation, and alkynylation". Science. 355 (6324): 499–503. doi:10.1126/science.aal5175. ISSN 0036-8075. PMC 5480404. PMID 28154075.
- ^ He, Jian; Shao, Qian; Wu, Qingfeng; Yu, Jin-Quan (2017-03-08). "Pd(II)-Catalyzed Enantioselective C(sp 3 )–H Borylation". Journal of the American Chemical Society. 139 (9): 3344–3347. doi:10.1021/jacs.6b13389. ISSN 0002-7863. PMID 28209055.
- ^ Wu, Qing-Feng; Wang, Xiao-Bing; Shen, Peng-Xiang; Yu, Jin-Quan (2018-03-02). "Enantioselective C–H Arylation and Vinylation of Cyclobutyl Carboxylic Amides". ACS Catalysis. 8 (3): 2577–2581. doi:10.1021/acscatal.8b00069. ISSN 2155-5435. PMC 5844484. PMID 29531850.
- ^ Shao, Qian; Wu, Qing-Feng; He, Jian; Yu, Jin-Quan (2018-04-25). "Enantioselective γ-C(sp 3 )–H Activation of Alkyl Amines via Pd(II)/Pd(0) Catalysis". Journal of the American Chemical Society. 140 (16): 5322–5325. doi:10.1021/jacs.8b01094. ISSN 0002-7863. PMC 5927623. PMID 29629766.
- ^ Shen, Peng-Xiang; Hu, Liang; Shao, Qian; Hong, Kai; Yu, Jin-Quan (2018-05-30). "Pd(II)-Catalyzed Enantioselective C(sp 3 )–H Arylation of Free Carboxylic Acids". Journal of the American Chemical Society. 140 (21): 6545–6549. doi:10.1021/jacs.8b03509. ISSN 0002-7863. PMC 6038808. PMID 29741883.
- ^ Zhuang, Zhe; Yu, Chang-Bin; Chen, Gang; Wu, Qing-Feng; Hsiao, Yi; Joe, Candice L.; Qiao, Jennifer X.; Poss, Michael A.; Yu, Jin-Quan (2018-08-15). "Ligand-Enabled β-C(sp 3 )–H Olefination of Free Carboxylic Acids". Journal of the American Chemical Society. 140 (32): 10363–10367. doi:10.1021/jacs.8b06527. ISSN 0002-7863. PMC 6524951. PMID 30029574.
- ^ Zhuang, Zhe; Yu, Jin-Quan (2020-07-15). "Pd(II)-Catalyzed Enantioselective γ-C(sp 3 )–H Functionalizations of Free Cyclopropylmethylamines". Journal of the American Chemical Society. 142 (28): 12015–12019. doi:10.1021/jacs.0c04801. ISSN 0002-7863. PMC 7654567. PMID 32605367.
- ^ Wang, Peng; Li, Gen-Cheng; Jain, Pankaj; Farmer, Marcus E.; He, Jian; Shen, Peng-Xiang; Yu, Jin-Quan (2016-10-26). "Ligand-Promoted meta -C–H Amination and Alkynylation". Journal of the American Chemical Society. 138 (42): 14092–14099. doi:10.1021/jacs.6b08942. ISSN 0002-7863. PMC 5513786. PMID 27712063.
- ^ Wang, Peng; Verma, Pritha; Xia, Guoqin; Shi, Jun; Qiao, Jennifer X.; Tao, Shiwei; Cheng, Peter T. W.; Poss, Michael A.; Farmer, Marcus E.; Yeung, Kap-Sun; Yu, Jin-Quan (November 2017). "Ligand-accelerated non-directed C–H functionalization of arenes". Nature. 551 (7681): 489–493. doi:10.1038/nature24632. ISSN 1476-4687. PMC 5726549. PMID 29168802.
- ^ Li, Zhen; Wang, Zhen; Chekshin, Nikita; Qian, Shaoqun; Qiao, Jennifer X.; Cheng, Peter T.; Yeung, Kap-Sun; Ewing, William R.; Yu, Jin-Quan (2021-06-25). "A tautomeric ligand enables directed C‒H hydroxylation with molecular oxygen". Science. 372 (6549): 1452–1457. doi:10.1126/science.abg2362. ISSN 0036-8075. PMC 8622180. PMID 34840353.
- ^ Wang, Zhen; Hu, Liang; Chekshin, Nikita; Zhuang, Zhe; Qian, Shaoqun; Qiao, Jennifer X.; Yu, Jin-Quan (2021-12-03). "Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C–H activation". Science. 374 (6572): 1281–1285. doi:10.1126/science.abl3939. ISSN 0036-8075. PMC 9084903. PMID 34762490.
- ^ Mal, Dipakranjan; Jana, Amit Kumar; Mitra, Prithiba; Ghosh, Ketaki (2011-05-06). "Benzannulation for the Regiodefined Synthesis of 2-Alkyl/Aryl-1-naphthols: Total Synthesis of Arnottin I". The Journal of Organic Chemistry. 76 (9): 3392–3398. doi:10.1021/jo2003677. ISSN 0022-3263. PMID 21405112.
- ^ Pospíšil, Jiří; Müller, Christoph; Fürstner, Alois (2009-06-08). "Total Synthesis of the Aspercyclides". Chemistry - A European Journal. 15 (24): 5956–5968. doi:10.1002/chem.200802681. PMID 19418521.
- ^ Wang, Hui-Hong; Wang, Xiao-Dong; Cao, Fei; Gao, Wei-Wei; Ma, Shu-Meng; Li, Zhao; Deng, Xue-Mei; Shi, Tao; Wang, Zhen (2021-01-05). "Application of palladium-catalyzed aryl C–H alkylation in total synthesis of (−)-berkelic acid". Organic Chemistry Frontiers. 8 (1): 82–86. doi:10.1039/D0QO01003K. ISSN 2052-4129. S2CID 228922066.
- ^ a b Zhang, Meng Yao; Barrow, Russell A. (2018-06-15). "Total Synthesis of Boletopsin 11 Enabled by Directed ortho -C(sp 2 )–H Arylation". The Journal of Organic Chemistry. 83 (12): 6776–6782. doi:10.1021/acs.joc.8b00792. ISSN 0022-3263. PMID 29792705.
- ^ Zhang, Zhongyin; Wang, Jinxin; Li, Jian; Yang, Fan; Liu, Guodu; Tang, Wenjun; He, Weiwei; Fu, Jian-Jun; Shen, Yun-Heng; Li, Ang; Zhang, Wei-Dong (2017-04-19). "Total Synthesis and Stereochemical Assignment of Delavatine A: Rh-Catalyzed Asymmetric Hydrogenation of Indene-Type Tetrasubstituted Olefins and Kinetic Resolution through Pd-Catalyzed Triflamide-Directed C–H Olefination". Journal of the American Chemical Society. 139 (15): 5558–5567. doi:10.1021/jacs.7b01718. ISSN 0002-7863. PMID 28271887.
- ^ Leal, Raul A.; Bischof, Caroline; Lee, Youjin V.; Sawano, Shota; McAtee, Christopher C.; Latimer, Luke N.; Russ, Zachary N.; Dueber, John E.; Yu, Jin‐Quan; Sarpong, Richmond (2016-09-19). "Application of a Palladium‐Catalyzed C−H Functionalization/Indolization Method to Syntheses of cis ‐Trikentrin A and Herbindole B". Angewandte Chemie International Edition. 55 (39): 11824–11828. doi:10.1002/anie.201605475. ISSN 1433-7851. PMID 27570932. S2CID 7847026.
- ^ Rosen, Brandon R.; Simke, Leah R.; Thuy-Boun, Peter S.; Dixon, Darryl D.; Yu, Jin-Quan; Baran, Phil S. (2013-07-08). "C—H Functionalization Logic Enables Synthesis of (+)-Hongoquercin A and Related Compounds". Angewandte Chemie International Edition. 52 (28): 7317–7320. doi:10.1002/anie.201303838. PMID 23740529.
- ^ Hong, Benke; Li, Chao; Wang, Zhen; Chen, Jie; Li, Houhua; Lei, Xiaoguang (2015-09-23). "Enantioselective Total Synthesis of (−)-Incarviatone A". Journal of the American Chemical Society. 137 (37): 11946–11949. doi:10.1021/jacs.5b08551. ISSN 0002-7863. PMID 26371964.
- ^ a b Bedell, T. Aaron; Hone, Graham A. B.; Valette, Damien; Yu, Jin-Quan; Davies, Huw M. L.; Sorensen, Erik J. (2016-07-11). "Rapid Construction of a Benzo-Fused Indoxamycin Core Enabled by Site-Selective C−H Functionalizations". Angewandte Chemie International Edition. 55 (29): 8270–8274. doi:10.1002/anie.201602024. PMID 27206223.
- ^ Wang, Dong-Hui; Engle, Keary M.; Shi, Bing-Feng; Yu, Jin-Quan (2010-01-15). "Ligand-Enabled Reactivity and Selectivity in a Synthetically Versatile Aryl C–H Olefination". Science. 327 (5963): 315–319. doi:10.1126/science.1182512. ISSN 0036-8075. PMC 2879878. PMID 19965380.
- ^ Kimura, Shingo; Kobayashi, Satoshi; Kumamoto, Takuya; Akagi, Aki; Sato, Naomi; Ishikawa, Tsutomu (April 2011). "Syntheses of Prekinamycin and a Tetracyclic Quinone from Common Synthetic Intermediates". Helvetica Chimica Acta. 94 (4): 578–591. doi:10.1002/hlca.201000296.
- ^ a b Wang, Dong-Hui; Yu, Jin-Quan (2011-04-20). "Highly Convergent Total Synthesis of (+)-Lithospermic Acid via a Late-Stage Intermolecular C−H Olefination". Journal of the American Chemical Society. 133 (15): 5767–5769. doi:10.1021/ja2010225. ISSN 0002-7863. PMC 3085405. PMID 21443224.
- ^ Davoren, Jennifer E.; Garnsey, Michelle; Pettersen, Betty; Brodney, Michael A.; Edgerton, Jeremy R.; Fortin, Jean-Philippe; Grimwood, Sarah; Harris, Anthony R.; Jenkinson, Stephen; Kenakin, Terry; Lazzaro, John T.; Lee, Che-Wah; Lotarski, Susan M.; Nottebaum, Lisa; O'Neil, Steven V. (2017-08-10). "Design and Synthesis of γ- and δ-Lactam M 1 Positive Allosteric Modulators (PAMs): Convulsion and Cholinergic Toxicity of an M 1 -Selective PAM with Weak Agonist Activity". Journal of Medicinal Chemistry. 60 (15): 6649–6663. doi:10.1021/acs.jmedchem.7b00597. ISSN 0022-2623. PMID 28598634.
- ^ Ye, Shengqing; Yang, Weibo; Coon, Timothy; Fanning, Dewey; Neubert, Tim; Stamos, Dean; Yu, Jin-Quan (2016-03-24). "N-Heterocyclic Carbene Ligand-Enabled C(sp 3 )−H Arylation of Piperidine and Tetrahydropyran Derivatives". Chemistry - A European Journal. 22 (14): 4748–4752. doi:10.1002/chem.201600191. PMC 4805524. PMID 26841330.
This article needs additional or more specific categories. (June 2023) |
