Lurtotecan
Appearance
| |
| Names | |
|---|---|
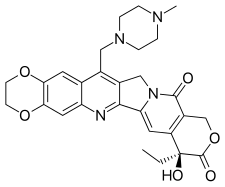
| Preferred IUPAC name
(8S)-8-Ethyl-8-hydroxy-15-[(4-methylpiperazin-1-yl)methyl]-2,3-dihydro-11H-[1,4]dioxino[2,3-g]pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-9,12(8H,14H)-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H30N4O6 | |
| Molar mass | 518.561 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lurtotecan is a semi-synthetic analog of camptothecin with antineoplastic activity. Liposomal lurtotecan was in clinical trials as a treatment for topotecan-resistant ovarian cancer,[1] but was discontinued.[2]
Synthesis
[edit]
- Heck reaction
- Mitsunobu reaction
- Potassium osmate
- Sharpless asymmetric dihydroxylation
- Swern oxidation
References
[edit]- ^ Seiden, MV; Muggia, F; Astrow, A; Matulonis, U; Campos, S; Roche, M; Sivret, J; Rusk, J; Barrett, E (April 2004). "A Phase II Study of Liposomal Lurtotecan (OSI-211) in Patients with Topotecan Resistant Ovarian Cancer". Gynecologic Oncology. 93 (1): 229–32. doi:10.1016/j.ygyno.2003.12.037. PMID 15047241.
- ^ "Liposomal lurtotecan (OSI 211) on AdisInsight". Adis Insight. Springer International Publishing AG. Retrieved 15 July 2016.
- ^ Fang, F. G.; Bankston, D. D.; Huie, E. M.; Ross Johnson, M.; Kang, M. C.; Lehoullier, C. S.; Lewis, G. C.; Lovelace, T. C.; Lowery, M. W.; McDougald, D. L.; Meerholz, C. A.; Partridge, J. J.; Sharp, M. J.; Xie, S. (1997). "Convergent catalytic asymmetric synthesis of camptothecin analog GI147211C". Tetrahedron. 53 (32): 10953. doi:10.1016/S0040-4020(97)00357-8.
