Hyocholic acid
| |
| Names | |
|---|---|
| IUPAC name
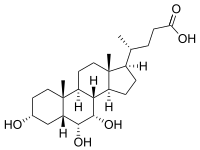
3α,6α,7α-Trihydroxy-5β-cholan-24-oic acid
| |
| Systematic IUPAC name
(4R)-4-[(1R,3aS,3bS,4S,5R,5aR,7R,9aR,9bS,11aR)-4,5,7-Trihydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]pentanoic acid | |
| Other names
γ-Muricholic acid; Iocholic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.008.124 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H40O5 | |
| Molar mass | 408.579 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hyocholic acid or 3α,6α,7α-trihydroxy-5β-cholan-24-oic acid is a bile acid found as one of the main forms in pig,[1] and at low concentrations in other species including humans.[2][3] Hyocholic acid differs from the primary bile acids found in humans by having a third hydroxyl group in the α-conformation at the 6-position, unlike cholic acid, which has a 12-hydroxyl, and chenodeoxycholic acid which has neither a 6- or 12-hydroxyl. It also differs from the muricholic acids found in rodents, as they are 6β-hydroxylated, and can have the 7-hydroxyl in either the α- or β- positions, forming α- or β-muricholic acids.
Hyocholic acid is conjugated in the liver before secretion with taurine or with glycine to give taurohyocholate or glycohyocholates. Bacterial 7α-dehydroxylation in the colon produces the secondary bile acid, hyodeoxycholic acid. Epimerization of the 7-hydroxyl to the β-position is found in ω-muricholic acid (also known as β-hyocholic acid).[4]
The enzyme responsible for the 6-hydroxylation reaction of chenodeoxycholic acid in the pig is the cytochrome P450 CYP4A21.[5]
Hyocholic acid can be found in humans with cholestasis[6] and may be increased after sleeve gastrectomy for obesity.[7]
References
[edit]- ^ Haslewood, GA (June 1971). "Bile salts of germ-free domestic fowl and pigs". The Biochemical Journal. 123 (1): 15–8. doi:10.1042/bj1230015. PMC 1176895. PMID 5128663.
- ^ Bathena, SP; Mukherjee, S; Olivera, M; Alnouti, Y (30 December 2013). "The profile of bile acids and their sulfate metabolites in human urine and serum". Journal of Chromatography B. 942–943: 53–62. doi:10.1016/j.jchromb.2013.10.019. PMID 24212143.
- ^ Russell DW (2003). "The enzymes, regulation, and genetics of bile acid synthesis". Annu. Rev. Biochem. 72: 137–74. doi:10.1146/annurev.biochem.72.121801.161712. PMID 12543708.
- ^ Hofmann, AF; Sjövall, J; Kurz, G; Radominska, A; Schteingart, CD; Tint, GS; Vlahcevic, ZR; Setchell, KD (April 1992). "A proposed nomenclature for bile acids". Journal of Lipid Research. 33 (4): 599–604. doi:10.1016/S0022-2275(20)41624-4. PMID 1527482.
- ^ Lundell, K; Hansson, R; Wikvall, K (30 March 2001). "Cloning and expression of a pig liver taurochenodeoxycholic acid 6alpha-hydroxylase (CYP4A21): a novel member of the CYP4A subfamily". The Journal of Biological Chemistry. 276 (13): 9606–12. doi:10.1074/jbc.M006584200. PMID 11113117.
- ^ van Berge Henegouwen, GP; Brandt, KH; Eyssen, H; Parmentier, G (November 1976). "Sulphated and unsulphated bile acids in serum, bile, and urine of patients with cholestasis". Gut. 17 (11): 861–9. doi:10.1136/gut.17.11.861. PMC 1411206. PMID 1001976.
- ^ Kindel, TL; Krause, C; Helm, MC; McBride, CL; Oleynikov, D; Thakare, R; Alamoudi, J; Kothari, V; Alnouti, Y; Kohli, R (February 2018). "Increased glycine-amidated hyocholic acid correlates to improved early weight loss after sleeve gastrectomy". Surgical Endoscopy. 32 (2): 805–812. doi:10.1007/s00464-017-5747-y. PMC 5844265. PMID 28779240.
