Granadaene
| |
| Names | |
|---|---|
| IUPAC name
(2S)-5-Amino-2-[[(2E,4E,6E,8E,10E,12E,14E,16E,18E,20E,22E,24E)-27-[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoctacosa-2,4,6,8,10,12,14,16,18,20,22,24-dodecaenoyl]amino]pentanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C39H52N2O8 | |
| Molar mass | 676.851 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
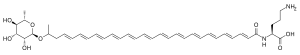
Granadaene is the trivial name of a polyene of non-isoprenoid biosynthetic origin, that constitutes the red pigment characteristic of Streptococcus agalactiae (group B streptococcus).
Characteristics
[edit]Granadaene contains a conjugated system made up of a linear chain of 12 conjugated carbon-carbon double bonds which is connected to the amino acid ornithine at one end and the sugar rhamnose at the other.[1][2] Granadaene contains 12 conjugated double bonds, a feature which is unprecedented among non-isoprenoid pigments.
Granadaene is dark red, odorless, insoluble in water, methanol, ethanol, diethyl ether, acetone, hexane, dimethyl sulfoxide (DMSO), acetonitrile, tetrahydrofuran, chloroform, and in most solvents, it is soluble in DMSO–0.1% trifluoroacetic acid (TFA).[1] Granadaene, can be extracted from cultures of S.agalactiae in granada broth (granada medium without agar) with 0.1 M potassium hydroxide (KOH) and purified by size-exclusion chromatography on Sephadex LH using DMSO–0.1%TFA.[1]


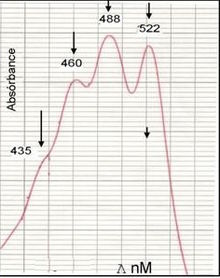

The fine-structure of the ultraviolet-visible absorption spectrum of the granadaene (in DMSO/TFA) is almost identical to that of a carotene with a similar conjugated system of 12 double bonds (e.g. alpha-carotene), which is why the GBS pigment was considered to be a carotene for many years.[3]
Non-terpenoid polyenes up to 10 conjugated double bonds are also a bizarre class of biologically natural products (laetiporic acids) found in the basidiomycete Laetiporus sulphureus.[4]
Granadaene and S.agalactiae detection and identification
[edit]Production of the red pigment granadaene is a phenotypic trait specific to β-hemolytic GBS, and because of that, detection of red colonies from clinical samples, when cultivated on granada medium, allows the straightforward identification of GBS.[5][6][7]
Biological relevance
[edit]Granadaene is an organic compound produced by S.agalactiae. It is the product of a metabolic pathway similar to that of biosynthesis of fatty acids. The enzymes necessary for the biosynthesis of granadaene in GBS are coded by a gene cluster of 12 genes, the cyl operon, Among the cyl operon, the cylE gene is required for pigment production, and transcription of cyl genes is regulated by the CovR/S two-component system,[8] and a pathway for the pigment biosynthesis requiring all the genes of the cyl operon has been proposed.
Like the biosynthesis of the pigment, the hemolytic activity also requires in GBS the 12 genes of the cyl operon.[9][10] Moreover,it has been proposed that granadaene is indeed the hemolysin of S.agalactiae
The pigment is localized, in GBS, in the cell membrane,[3] where it could play a role in membrane stabilization, similar to the role of carotenes in other bacterial membranes.[11] In addition to S.agalactiae the presence of granadaene and the cyl genes has been reported in pigmented Acidipropionibacterium spp. (former Propionibacterium) as A.jensenii, A.thoenii, and A.virtanenii,[12] where it can cause defects such as red spots in some cheeses.[13] Probably granadaene is also present in other related species such as Pseudopropionibacterium rubrum.[8][13][14] Granadaene is also produced by strains of Lactocococcus garvieae/petaury/formosensis group where the cyl cluster is also present.[15]
The cyl genes have been cloned in Lactococcus lactis (a non-hemolytic non-pigmented Gram-positive bacterium) and the expression of the GBS cyl operon conferred hemolysis, pigmentation, and cytotoxicity to Lactococcus lactis. Proving that the expression of the genes of the cyl operon is sufficient for Granadaene production in a heterologous host.[16]
Granadaene and GBS Virulence
[edit]The hemolytic activity of granadene is strongly linked to the length of its polyene chain.[17][18]
It has been proposed that granadaene is indeed the hemolysin of S.agalactiae, the GBS hemolysin is a broad-spectrum cytolysin able to destroy many eukaryotic cells, including platelets. Because of this, granadaene is considered an important virulence factor for GBS.[8][19][20][21][22][23][24][25]
Nevertheless, 1–5% of GBS strains are non-hemolytic and do not produce pigment. [8] These non-hemolytic and non-pigmented GBS strains (lacking pigment and hemolysin) are considered less virulent because of that.[19][21][26][27]
References
[edit]- ^ a b c Rosa-Fraile M, Rodríguez-Granger J, Haidour-Benamin A, Cuerva JM, Sampedro A (2006). "Granadaene: Proposed Structure of the Group B Streptococcus Polyenic Pigment". Appl Environ Microbiol. 72 (9): 6367–6370. Bibcode:2006ApEnM..72.6367R. doi:10.1128/aem.00756-06. PMC 1563658. PMID 16957264.
- ^ Paradas M, Jurado R, Haidour A, Rodríguez Granger J, Sampedro Martínez A, de la Rosa Fraile M, Robles R, Justicia J, Cuerva JM (2012). "Clarifying the structure of granadaene: Total synthesis of related analogue - granadaene and confirmation of its absolute stereochemistry". Bioorg Med Chem. 20 (22): 6655–6661. doi:10.1016/j.bmc.2012.09.017. PMID 23043725.
- ^ a b Merrit K, Jacobs NJ (1978). "Characterization and Incidence of Pigment Production by Human Clinical Group B Streptococci". J Clin Microbiol. 8 (1): 105–107. doi:10.1128/jcm.8.1.105-107.1978. PMC 275130. PMID 353069.
- ^ Seibold PS. Lenz C. Gressler M. Hoffmeister D. (2020). "The Laetiporus polyketide synthase LpaA produces a series of antifungal polyenes". J Antibiot (Tokyo). 73 (10): 711–720. doi:10.1038/s41429-020-00362-6. PMC 7473843. PMID 32820242.
- ^ M Rosa-Fraile, J Rodriguez-Granger, M Cueto-Lopez, A Sampedro, E B Gaye, J M Haro, A Andreu (1999). "Use of Granada medium to detect group B streptococcal colonization in pregnant women". J Clin Microbiol. 37 (8): 2674–2677. doi:10.1128/JCM.37.8.2674-2677.1999. PMC 85311. PMID 10405420.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Verani JR, McGee L, Schrag SJ (2010). "Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010" (PDF). MMWR Recomm Rep. 59(RR-10): 1–32.
- ^ Filkins L , Hauser J, Robinson-Dunn B, Tibbetts R, Boyanton B , Revell P. "Guidelines for the Detection and Identification of Group B Streptococcus. 2021". American Society for Microbiology. Retrieved 23 May 2023.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Rosa-Fraile M, Dramsi S, Spellerberg B (2014). "Group B streptococcal haemolysin and pigment, a tale of twins". FEMS Microbiol. Rev. 38 (5): 932–946. doi:10.1111/1574-6976.12071. PMC 4315905. PMID 24617549.
- ^ Spellerberg B, Pohl B, Haase G, Martin S, Weber-Heynemann J, Lütticken R (1999). "Identification of genetic determinants for the hemolytic activity of Streptococcus agalactiae by ISS1 transposition". J. Bacteriol. 181 (10): 3212–3219. doi:10.1128/JB.181.10.3212-3219.1999. PMC 93778. PMID 10322024.
- ^ Spellerberg B, Martin S, Brandt C, Lütticken R (2000). "The cyl genes of Streptococcus agalactiae are involved in the production of pigment". FEMS Microbiol. Lett. 188 (2): 125–128. doi:10.1111/j.1574-6968.2000.tb09182.x. PMID 10913694.
- ^ Taylor RF (1984). "Bacterial tryterpenoids". Microbiol. Rev. 48 (3): 181–198. doi:10.1128/MMBR.48.3.181-198.1984. PMC 373008. PMID 6387426.
- ^ Deptula P, Loivamaa L , Smolander OP, Laine P , Roberts RJ, Piironen V, Paulin L, Savijoki K, Auvinen P, Varmanen P (2019). "Red-Brown Pigmentation of Acidipropionibacterium jensenii Is Tied to Haemolytic Activity and cyl-Like Gene Cluster". Microorganisms 7: 512. https://doi.org/10.3390/microorganisms7110512 |https://pmc.ncbi.nlm.nih.gov/articles/PMC6920887/%7C
- ^ a b Vanberg C, Lutnaes BF, Langsrud T, Nees IF, Holo H (2007). "Propionibacterium jensenii produces the polyene pigment granadaene and has hemolytic properties similar to those of Streptococcus agalactiae". Appl Environ Microbiol. 73 (17): 5501–5506. Bibcode:2007ApEnM..73.5501V. doi:10.1128/AEM.00545-07. PMC 2042088. PMID 17630313.
- ^ Masanori Saito, Noriko Shinozaki-Kuwahara, Osamu Tsudukibashi, Tomomi Hashizume-Takizawa, Ryoki Kobayashi, Tomoko Kurita-Ochiai (2018). "Pseudopropionibacterium rubrum sp. nov., a novel red-pigmented species isolated from human gingival sulcus". Microbiol Immunol. 62 (6): 388–394. doi:10.1111/1348-0421.12592. PMID 29687917. S2CID 22322865.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Neuzil-Bunesova V, Ramirez Garcia A, Modrackova N, Makovska M, Sabolova M, Spröer C, Bunk B, Blom J, Schwab C (2022). "Feed Insects as a Reservoir of Granadaene-Producing Lactococci". Front. Microbiol. 13: Art 848490. doi:10.3389/fmicb.2022.848490. PMC 9125021. PMID 35615513.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Armistead B, Whidbey C, Iyer LM, Herrero-Foncubierta P, Quach P, Haidour A, Aravind L, Cuerva JM, Jaspan HB, Rajagopal L. (2020). "The cyl Genes Reveal the Biosynthetic and Evolutionary Origins of the Group B Streptococcus Hemolytic Lipid, Granadaene". Front. Microbiol. 10: 3123. doi:10.3389/fmicb.2019.03123. PMC 6985545. PMID 32038561.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kristanc L, Božič B, Jokhadar ŠZ, Dolenc MS, Gomišček G. (2019). "The pore-forming action of polyenes: From model membranes to living organisms". Biochim Biophys Acta Biomembr. 186 (2): 418–430. doi:10.1016/j.bbamem.2018.11.006. PMID 30458121. S2CID 53735664.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Armistead B, Herrero-Foncubierta P, Coleman M, Quach P, Whidbey C, Justicia J, Tapia R, Casares R, Millán A, Haidour A, Rodriguez Granger J, Vornhagen J, Santana-Ufret V, Merillat S, Waldorf KA, Cuerva JM, Rajagopal L (2020). "Lipid analogs reveal features critical for hemolysis and diminish granadaene mediated Group B Streptococcus infection". Nature Communications. 11 (1): 1502. Bibcode:2020NatCo..11.1502A. doi:10.1038/s41467-020-15282-0. PMC 7083881. PMID 32198389. Retrieved 30 May 2022.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Waldorf KM, Rajagopal L (2013). "A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta" (PDF). J Exp Med. 210 (6): 1265–1281. doi:10.1084/jem.20122753. PMC 3674703. PMID 23712433.
- ^ Liu Y, Liu J. (2022). "Group B Streptococcus: Virulence Factors and Pathogenic Mechanism". Microorganisms. 15 (12): 2483. doi:10.3390/microorganisms10122483. PMC 9784991. PMID 36557736.
- ^ a b Whidbey C, Vornhagen J, Gendrin C, Boldenow E, Samson JM, Doering K, Ngo L, Ezekwe EA Jr, Gundlach JH, Elovitz MA, Liggitt D, Duncan JA, Adams Waldorf KM, Rajagopal L (2015). "A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury". EMBO Mol. Med. 7 (4): 488–505. doi:10.15252/emmm.201404883. PMC 4403049. PMID 25750210.
- ^ Armistead B, Oler E, Adams Waldorf K, Rajagopal L. (2019). "The Double Life of Group B Streptococcus: Asymptomatic Colonizer and Potent Pathogen". J Mol Biol. 431 (16): 2914–2931. doi:10.1016/j.jmb.2019.01.035. PMC 6646060. PMID 30711542.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Armistead B, Herrero-Foncubierta P, Coleman M, Quach P, Whidbey C, Justicia J, Tapia R, Casares R, Millán A, Haidour A, Granger JR, Vornhagen J, Santana-Ufret V, Merillat S, Adams Waldorf K, Cuerva JM, Rajagopal L. (2020). "Lipid analogs reveal features critical for hemolysis and diminish granadaene mediated Group B Streptococcus infection". Nat. Commun. 11 (1): 1502. Bibcode:2020NatCo..11.1502A. doi:10.1038/s41467-020-15282-0. PMC 7083881. PMID 32198389.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Huebner EM, Gudjónsdóttir MJ, Dacanay MB, Nguyen S, Brokaw A, Sharma K, Elfvin A, Hentz E, Rivera YR, Burd N, Shivakumar M, Coler B, Li M, Li A, Munson J, Orvis A, Coleman M, Jacobsson B, Rajagopal L, Adams Waldorf KM. (2022). "Virulence, phenotype and genotype characteristics of invasive group B Streptococcus isolates obtained from Swedish pregnant women and neonates". Ann Clin Microbiol Antimicrob. 21 (1): 43. doi:10.1186/s12941-022-00534-2. PMC 9560721. PMID 36229877.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jahn, K.; Shumba, P.; Quach, P.; Müsken, M.; Wesche, J.; Greinacher, A.; Rajagopal, L.; Hammerschmidt, S.; Siemens, N. (2022). "Group B Streptococcal Hemolytic Pigment Impairs Platelet Function in a Two-Step Process". Cells. 11 (10): 1637. doi:10.3390/cells11101637. PMC 9139542. PMID 35626674.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rodriguez-Granger J, Spellerberg B, Asam D, Rosa-Fraile M (2015). "Non-haemolytic and non-pigmented group b streptococcus, an infrequent cause of early onset neonatal sepsis". Pathogens and Disease. 73 (9): ftv089. doi:10.1093/femspd/ftv089. PMC 4626576. PMID 26449711.
- ^ Armistead B, Quach P, Snyder JM, Santana-Ufret V, Furuta A, Brokaw A, Rajagopal L. (2020). "Hemolytic membrane vesicles of Group B Streptococcus promote infection". The Journal of Infectious Diseases. jiaa548 (8): 1488–1496. doi:10.1093/infdis/jiaa548. PMC 8064051. PMID 32861213.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
