Gerbrand Ceder
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Gerbrand Ceder | |
|---|---|
 | |
| Alma mater | KU Leuven (BA) University of California, Berkeley (PhD) |
| Awards | 2017 National Academy of Engineering 2016 International Battery Award Research 2016 MRS Materials Theory Award 2015 MRS Fellow 2009 MRS Gold Medal 2007 MIT School of Engineering Graduate Teaching Award 2004 ECS Battery Research Award |
| Scientific career | |
| Fields | Computational materials science Computational materials design |
| Institutions | University of California, Berkeley |
| Doctoral advisor | Didier de Fontaine |
| Doctoral students | |
| Website | ceder |
Gerbrand Ceder is a Belgian–American scientist who is a professor and the Samsung Distinguished Chair in Nanoscience and Nanotechnology Research at the University of California, Berkeley.[1][2] He has a joint appointment as a senior faculty scientist in the Materials Sciences Division of Lawrence Berkeley National Laboratory. He is notable for his pioneering research in high-throughput computational materials design, and in the development of novel lithium-ion battery technologies. He is co-founder of the Materials Project, an open-source online database of ab initio calculated material properties, which inspired the Materials Genome Initiative[3] by the Obama administration in 2011. He was previously the founder and CTO of Pellion Technologies (having initially been CEO), which aimed to commercialize magnesium-ion batteries. In 2017 Gerbrand Ceder was elected a member of the National Academy of Engineering, "For the development of practical computational materials design and its application to the improvement of energy storage technology."[4]
Career
[edit]Gerbrand Ceder received an engineering degree in Metallurgy and Applied Materials Science from the KU Leuven, Belgium, in 1988, and a PhD in materials science from the University of California at Berkeley in 1991, at which time he joined the faculty at Massachusetts Institute of Technology (MIT). He was the R.P. Simmons Professor of Materials Science and Engineering at the Massachusetts Institute of Technology for 25 years, after which he moved back to the U. C. Berkeley, where he remains. His research group focuses on the use of computational modeling to design novel materials for energy generation and storage, including battery cathodes, hydrogen storage materials, thermoelectrics, and electrodes for solar photoelectrochemical water-splitters. His group also designs, synthesizes and characterizes novel lithium-ion and sodium-ion battery chemistries. He has published over 400 scientific papers in the fields of alloy theory, oxide phase stability, high-temperature superconductors, Li-battery materials, machine learning, and theory of materials synthesis, and holds 25 current or pending U.S. patents.
Research
[edit]Li-ion Batteries Cathode Materials
[edit]In 2009, ByungWoo Kang and Gerbrand Ceder demonstrated that the lithium-ion battery cathode material LiFePO4 could undergo ultrafast charging and discharging (~10 sec full discharge).[5]
In 2014, Jinhyuk Lee and Gerbrand Ceder demonstrated that when the Li content surpasses the percolation threshold, disordered rocksalt structures can deliver high discharge capacity (>300 mAh/g) and energy density (>1000 Wh/kg). The discovery opens up new opportunities for Nickel and cobalt free cathode materials for Li-ion batteries and significantly lowers the cost of cathode materials.[6]
Solid State Ionic Conductors
[edit]Gerbrand Ceder has made significant contributions in the field of ionic superconductors which have application as electrolytes in solid-state batteries. Most of Ceder's works involve the use of first-principles based computaional methods for understanding the atomic scale mechanism and design prinicples of conductors. In 2011, Yifei Mo, S.P. Ong and Gerbrand Ceder used ab-initio molecular dynamics simulations to clarify how the state-of-the art conductor (LGPS) is essentially a 3-dimensional conductor (as opposed to the previously presumed 1D conduction mechanism), which bypasses the inevitable detrimental effects of blocking defects observed in 1D conduction mechanisms. [7] In 2015, Ceder and his co-authors identified the overarching structural feature in many superionic conductors such as LGPS. The authors showed that the anion arrangement is the key factor in determining intrinsic Li-ion mobility and body-centered cubic (bcc) anion sublattice is most desirable for achieving high ionic conductivity, as it allows direct Li hops between adjacent tetrahedral sites. [8] In 2016, Ceder and co-authors presented a computational prediction, discovery and synthesis of which has a has a room temperature ionic conductivity of 0.4 mS cm−1, which rivals the conductivity of the best sodium sulfide solid electrolytes to date[9]
In 2022, Ceder and co-authors made a major breakthrough in understanding the structural features leading to high ionic conductivity in the relatively safer and more stable class of oxide conductors. The authors showed that oxides (which typically do not crystallize in the favorable BCC anion frameworks like sulfides), can promote super-ionic conductivity in anion frameworks with corner-sharing connectivity despite the poor screening ability of oxides compared to sulfides.[10]
In 2022, Gerbrand Ceder, Yan Zheng, Bin Ouyang, and co-authors demonstrated another mechanism for boosting ionic conductivity in oxide conductors. The authors showed how high-entropy compositions in Na super ionic conductor (NASICON) material and garnets can create local structural distortions that enhance alkali ion mobility.[11] In 2023, Ceder and co-authors outlined important design principles in NASICON materials, associating performance to features such as optimal Na content, polyanion chemistry, cation size and silicate content. [12] In 2024, Gerbrand Ceder and co-authors have demonstrated design strategies for Li superionic conductivity in fcc based oxides (traditionally not been considered suitable for solid-state electrolytes) by achieving cation over-stoichiometry in rocksalt-type lattices via excess Li in the composition.[13] Other recent significant contributions from Gerbrand in this field include detailed computational study of atomic scale mechanisms in [14] and the emerging clay-like superionic conductors[15].
Gerbrand Ceder has also made contributions in experimental efforts to identify failure mechanisms associated with solid-state electrolyte production. [16]
Autonomous Materials Discovery
[edit]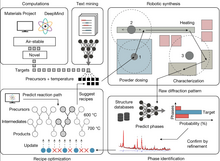
In November 2023, Ceder and colleagues introduced A-Lab, an autonomous laboratory for inorganic powder synthesis, integrating computations, machine learning, and robotics.[17] The work garnered significant attention[18][19] based on the claim it successfully synthesized 41 out of 58 novel compounds, primarily oxides and phosphates, over 17 days. The A-lab used graph neural networks trained on computational materials databases and literature-trained natural language models for initial synthesis recipe proposals, optimized through active learning based on thermodynamics.
In January of 2023, a preprint[20] scrutinized the accuracy of autonomous material discovery methods used in Szymanski et al. paper.[17] They argue the initial claim of 43 new materials via A-lab, faces issues with automated Rietveld analysis in X-ray diffraction and neglected material disorder, leading to Palgrave et al.'s conclusion that no new materials were discovered.[20] The preprint stresses the need for improved approaches to using AI tools and computational models. Prior to the preprint criticism by Palgrave online[21] was addressed by Ceder in a LinkedIn post refuting many critiques made against the A-lab paper.
Awards and honors
[edit]Professor Ceder is an elected Fellow of the Materials Research Society (MRS),[22] the American Physical Society (APS),[23] The Minerals, Metals & Materials Society (TMS),[24] and the Electrochemical Society (ECS).[25] In 2017, he was elected to the US National Academy of Engineering for "For the development of practical computational materials design and its application to the improvement of energy storage technology".[26][27] He is also a member of the American Academy of the Arts and Science (2022)[28][29] and a Fellow of the Royal Flemish Academy of Arts and Sciences (2016).[30]
As a young faculty member at MIT he received multiple awards for his work, including an National Science Foundation Early Career Award and the TMS Robert Lansing Hardy Award for from The Minerals, Metals & Materials Society for "exceptional promise for a successful career" (1996).[31][32] In 1999 he was also appointed to the Res Metallica Chair of his alma mater, the K.U. Leuven.
Professor Ceder's work on energy storage materials include the Battery Research Award from the Electrochemical Society in 2004,[33] the Research Award from the International Battery Association in 2017,[34] and in 2009 the Materials Research Society (MRS) Gold Medal "For pioneering the high-impact field of first-principles thermodynamics of batteries materials and for the development of high-power density Li battery compounds".[35][36]
For his work on developing materials theory and computational materials science he received the MRS Materials Theory Award in 2016,[37][38] and in 2019 the National Institute for Materials Science (NIMS – Japan) Award for Data-driven Materials Research.[39] In 2023 he was awarded the Hume Rothery Award from TMS for "seminal contributions to theory and predictive computational methods for complex multicomponent alloys and ceramic solid solutions, and pioneering advances for ab-initio materials design".[40] Most recently he was honored with the Charles Hatchett Award for the productive use of Nb in energy storage materials.[41] Other awards include the TMS Morris Cohen Award (2016)[42] and the Alexander M. Cruickshank Award at the 2015 Gordon Conference.
See also
[edit]References
[edit]- ^ "Gerbrand Ceder". Materials Science & Engineering. 2023. Retrieved 2024-03-08.
- ^ "Massachusetts Institute of Technology's Technology Review, Volume 102". Massachusetts Institute of Technology. 1999. Retrieved September 19, 2016.
- ^ "The Materials Genome Initiative". whitehouse.gov – via National Archives.
- ^ "National Academy of Engineering Elects 84 Members and 22 Foreign Members". NAE Website.
- ^ Kang, Byungwoo; Ceder, Gerbrand (2009). "Battery materials for ultrafast charging and discharging". Nature. 458 (7235): 190–193. Bibcode:2009Natur.458..190K. doi:10.1038/nature07853. PMID 19279634. S2CID 20592628.
- ^ Lee, Jinhyuk; Urban, Alexander; Su, Dong; Hautier, Geoffroy; Ceder, Gerbrand (2014). "Unlocking the Potential of Cation-Disordered Oxides for Rechargeable Lithium Batteries". Science. 343 (6170): 519–522. Bibcode:2014Sci...343..519L. doi:10.1126/science.1246432. PMID 24407480.
- ^ Mo, Yifei; Ong, Shyue Ping; Ceder, Gerbrand (2012). "First Principles Study of the Li10GeP2S12 Lithium Super Ionic Conductor Material". Chemistry of Materials. 24 (1): 15–17. doi:10.1021/cm203303y.
- ^ Wang, Yan; Richards, William Davidson; Ong, Shyue Ping; Miara, Lincoln J.; Kim, Jae Chul; Mo, Yifei; Ceder, Gerbrand (2015). "Design principles for solid-state lithium superionic conductors". Nature Materials. 14: 1026–1031. doi:10.1038/nmat4180.
- ^ Richards, William D.; Tsujimura, Tomoyuki; Miara, Lincoln J.; Wang, Yan; Kim, Jae Chul; Ong, Shyue Ping; Uechi, Ichiro; Suzuki, Naoki; Ceder, Gerbrand (2016). "Design and synthesis of the superionic conductor Na10SnP2S12". Nature Communications. 7. doi:10.1038/ncomms11009.
{{cite journal}}:|article=ignored (help) - ^ Jun, KyuJung; Sun, Yingzhi; Xiao, Yihan; Zeng, Yan; Kim, Ryounghee; Kim, Haegyeom; Miara, Lincoln J.; Im, Dongmin; Wang, Yan; Ceder, Gerbrand (2022). "Lithium superionic conductors with corner-sharing frameworks". Nature Materials. 21: 924–931. doi:10.1038/s41563-022-01286-w.
- ^ Zeng, Yan; Ouyang, Bin; Liu, Jue; Byeon, Young-Woon; Cai, Zijian; Miara, Lincoln J.; Wang, Yan; Ceder, Gerbrand (2022). "High-entropy mechanism to boost ionic conductivity". Science. 378 (6626): 1320–1324. doi:10.1126/science.abq1346.
- ^ Wang, Jingyang; He, Tanjin; Yang, Xiaochen; Cai, Zijian; Wang, Yan; Lacivita, Valentina; Kim, Haegyeom; Ouyang, Bin; Ceder, Gerbrand (2023). "Design principles for NASICON super-ionic conductors". Nature Communications. 14. doi:10.1038/s41467-023-40834-3.
{{cite journal}}:|article=ignored (help) - ^ Chen, Yu; Lun, Zhengyan; Zhao, Xinye; Koirala, Krishna Prasad; Li, Linze; Sun, Yingzhi; O’Keefe, Christopher A.; Yang, Xiaochen; Cai, Zijian; Wang, Chongmin; Ji, Huiwen; Grey, Clare P.; Ouyang, Bin; Ceder, Gerbrand (2024). "Unlocking Li superionic conductivity in face-centred cubic oxides via face-sharing configurations". Nature Materials. 23: 535–542. doi:10.1038/s41563-024-01742-9.
- ^ Wang, Jingyang; He, Tanjin; Yang, Xiaochen; Cai, Zijian; Wang, Yan; Lacivita, Valentina; Kim, Haegyeom; Ouyang, Bin; Ceder, Gerbrand (2023). "Design principles for NASICON super-ionic conductors". Nature Communications. 14. doi:10.1038/s41467-023-40834-3.
{{cite journal}}:|article=ignored (help) - ^ Gupta, Sunny; Yang, Xiaochen; Ceder, Gerbrand (2023). "What dictates soft clay-like lithium superionic conductor formation from rigid salts mixture". Nature Communications. 14. doi:10.1038/s41467-023-42484-3.
{{cite journal}}:|article=ignored (help) - ^ Diallo, Mouhamad S.; Shi, Tan; Zhang, Yaqian; Peng, Xinxing; Shozib, Imtiaz; Wang, Yan; Miara, Lincoln J.; Scott, Mary C.; Tu, Qingsong Howard; Ceder, Gerbrand (2024). "Effect of solid-electrolyte pellet density on failure of solid-state batteries". Nature Communications. 15. doi:10.1038/s41467-024-44626-2.
{{cite journal}}:|article=ignored (help) - ^ a b c Szymanski, Nathan J.; Rendy, Bernardus; Fei, Yuxing; Kumar, Rishi E.; He, Tanjin; Milsted, David; McDermott, Matthew J.; Gallant, Max; Cubuk, Ekin Dogus; Merchant, Amil; Kim, Haegyeom; Jain, Anubhav; Bartel, Christopher J.; Persson, Kristin; Zeng, Yan (2023-11-29). "An autonomous laboratory for the accelerated synthesis of novel materials". Nature. 624 (7990): 86–91. Bibcode:2023Natur.624...86S. doi:10.1038/s41586-023-06734-w. ISSN 1476-4687. PMC 10700133. PMID 38030721.
- ^ Peplow, Mark (2023-11-29). "Google AI and robots join forces to build new materials". Nature. doi:10.1038/d41586-023-03745-5. PMID 38030771.
- ^ Masse, Bryson (2023-12-04). "AI meets materials science: the promise and pitfalls of automated discovery". VentureBeat. Retrieved 2024-01-17.
- ^ a b Leeman, Josh, Challenges in high-throughput inorganic material prediction and autonomous synthesis.
- ^ Peplow, Mark (2023-12-12). "Robot chemist sparks row with claim it created new materials". Nature. doi:10.1038/d41586-023-03956-w.
- ^ "MRS Fellows". mrs.org.
- ^ "APS Fellows". aps.org.
- ^ "TMS 2018 Class f Fellows". newswise.com.
- ^ "ECS Fellows 2022". electrochem.org.
- ^ "National Academy of Engineering 2017". nae.edu.
- ^ "National Academy of Engineering Election Citation". nae.edu.
- ^ "American Academy of the Arts and Science Members 2022". amacad.org. 5 December 2022.
- ^ "American Academy of the Arts and Science Member - Gerbrand Ceder". amacad.org. 14 May 2024.
- ^ "Fellow of the Royal Flemish Academy of Arts and Sciences". mse.berkeley.edu. 29 October 2015.
- ^ "AIME Robert Lansing Hardy Award". The Minerals, Metals & Materials Society (TMS). Retrieved March 8, 2024.
- ^ "MIT Annual Report 1996". MIT Annual Reports. Retrieved March 8, 2024.
- ^ "Battery Division Research Award". The Electrochemical Society (ECS). Retrieved March 8, 2024.
- ^ "Research Award from the International Battery Association".
- ^ "2009 MRS Medal". news.mit.edu. 8 September 2009.
- ^ "Title of the document" (PDF). SpringerLink. doi:10.1557/mrs2009.195. Retrieved March 8, 2024.
- ^ "Materials Theory Award 2016". mrs.org.
- ^ "Gerbrand Ceder receives 2016 Materials Theory Award". MRS Bulletin. 41 (10). Springer: 813. October 10, 2016. Bibcode:2016MRSBu..41..813.. doi:10.1557/mrs.2016.233. Retrieved March 8, 2024.
- ^ "NIMS Award 2019". National Institute for Materials Science (NIMS). Retrieved March 8, 2024.
- ^ "TMS William Hume Rothery Award". The Minerals, Metals & Materials Society (TMS).
- ^ "Charles Hatchett Award". niobium.tech/en/.
- ^ "Morris Cohen Awards". The Minerals, Metals & Materials Society (TMS).
External links
[edit]- American materials scientists
- 21st-century American engineers
- American science writers
- Old University of Leuven alumni
- UC Berkeley College of Engineering alumni
- MIT School of Engineering faculty
- Living people
- American chief executives
- American chief technology officers
- 21st-century Belgian scientists
- 21st-century Belgian engineers
- Belgian emigrants to the United States
- Fellows of the American Physical Society
- Fellows of the Minerals, Metals & Materials Society



