Gassman indole synthesis
The Gassman indole synthesis is a series of chemical reactions used to synthesize substituted indoles by addition of an aniline and a ketone bearing a thioether substituent.
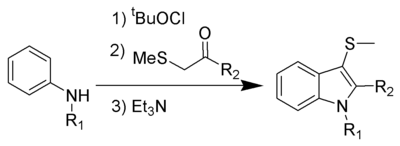
This is a one-pot chemical reaction, and none of the intermediates are isolated. R1 can be hydrogen or alkyl, while R2 works best with aryl, but can also be alkyl. Electron-rich anilines, such as 4-methoxyaniline, tend to fail in this reaction.
The 3-position thiomethyl group is often removed using Raney nickel to give the 3-H-indole.
Reaction mechanism
[edit]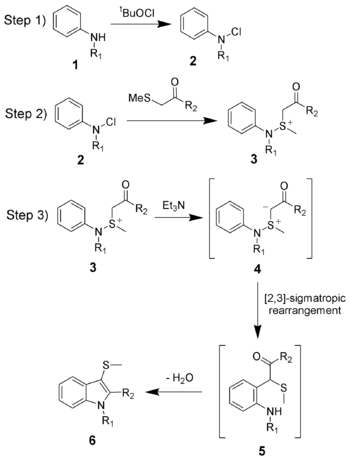
The reaction mechanism of the Gassman indole synthesis is divided among three steps.
The first step is the oxidation of the aniline 1 using tert-butyl hypochlorite (tBuOCl) to give the chloramine 2.
The second step is the addition of the keto-thioether to give the sulfonium ion 3, and is typically done at low temperatures (−78 °C).
The third and final step is the addition of a base, which in this case is triethylamine. Upon warming to room temperature, the base will deprotonate the sulfonium ion creating the sulfonium ylide 4, which quickly undergoes a [2,3]-sigmatropic rearrangement to give the ketone 5. The ketone 5 will undergo a facile condensation to give the desired 3-thiomethylindole 6.
References
[edit]- Gassman, P. G.; Gruetzmacher, G.; van Bergen, T. J. (1973). "Use of halogen-sulfide complexes in the synthesis of indoles, oxindoles, and alkylated aromatic amines". J. Am. Chem. Soc. 95 (19): 6508. doi:10.1021/ja00800a088.
- Gassman, P. G.; van Bergen, T. J.; Gilbert, D. P.; Cue, B. W. Jr. (1974). "General method for the synthesis of indoles". J. Am. Chem. Soc. 96 (17): 5495. doi:10.1021/ja00824a028.
- Gassman, P. G.; van Bergen, T. J. (1974). "Oxindoles. New, general method of synthesis". J. Am. Chem. Soc. 96 (17): 5508. doi:10.1021/ja00824a029.
- Gassman, P. G.; Gruetzmacher, G.; van Bergen, T. J. (1974). "Generation of azasulfonium salts from halogen-sulfide complexes and anilines. Synthesis of indoles, oxindoles, and alkylated aromatic amines bearing cation stabilizing substituents". J. Am. Chem. Soc. 96 (17): 5512. doi:10.1021/ja00824a030.
- Organic Syntheses, Coll. Vol. 6, p. 601; Vol. 56, p. 72 (Article)
