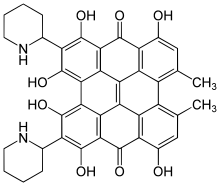
Fagopyrin
Appearance
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,3,4,6,8,13-Hexahydroxy-10,11-dimethyl-2,5-di(piperidin-2-yl)fenantro[1,10,9,8-opqra]peryleen-7,14-dion
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C40H34N2O8 | |
| Molar mass | 670.718 g·mol−1 |
| Appearance | Red pigment |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fagopyrin is a term used for several closely related naturally occurring substances in the buckwheat plant.[1] Their chemical structure contains a naphthodianthrone skeleton similar to that of hypericin.[2]
Fagopyrin is located almost exclusively in the cotyledons of the buckwheat herb. When ingested, fagopyrins cause sensitivity to light.[3]
References
[edit]- ^ Brockmann, Hans; Weber, Erhard; Sander, Elsbeth (1950). "Fagopyrin, ein photodynamischer Farbstoff aus Buchweizen (Fagopyrum esculentum)". Die Naturwissenschaften. 37 (2): 43. Bibcode:1950NW.....37...43B. doi:10.1007/BF00645366. S2CID 29975083.
- ^ Tavčar Benković, Eva; Žigon, Dušan; Friedrich, Miha; Plavec, Janez; Kreft, Samo (2014). "Isolation, analysis and structures of phototoxic fagopyrins from buckwheat". Food Chemistry. 143: 432–439. doi:10.1016/j.foodchem.2013.07.118. PMID 24054263.
- ^ Kreft, S.; Janeš, D.; Kreft, I. (2013). "The content of fagopyrin and polyphenols in common and tartary buckwheat sprouts". Acta Pharmaceutica. 63 (4): 553–60. doi:10.2478/acph-2013-0031. PMID 24451079.
