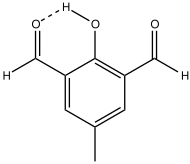
Diformylcresol
Appearance
| |
| Names | |
|---|---|
| IUPAC name
2-hydroxy-5-methylisophthalaldehyde
| |
| Other names
2,6-diformyl-4-methylphenol
2-hydroxy-5-methyl-1,3-benzenedicarboxaldehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.971 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H8O3 | |
| Molar mass | 164.160 g·mol−1 |
| Appearance | white solid |
| Density | 1.433 g/cm3[1] |
| Melting point | 113 °C (235 °F; 386 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diformylcresol is an organic compound with the formula CH3C6H2(CHO)2OH. The 2,6-diformyl derivative of p-cresol is the most common isomer and is a white solid at room temperature.
Diformylcresol condenses with amines to give diimines that are widely studied as binucleating ligands.[2][3]
Synthesis
[edit]Formyl groups (aldehydes) are fairly strong deactivating groups for electrophilic aromatic substitution reactions, hence double-addition to a phenol requires forcing conditions. Diformylcresol may be prepared from p-cresol by the Reimer-Tiemann reaction or the Duff reaction.[4]
The corresponding reaction of phenol would be expected to lead to formylation of the 4-position vs 2,6-selectivity.[5]
Related compounds
[edit]- salicylaldehyde, a phenol with only one flanking formyl group
References
[edit]- ^ Habarurema, Gratien; Gerber, Thomas I. A.; Hosten, Eric; Betz, Richard (2014). "Redetermination of the crystal structure of 2,6-diformyl-4-methylphenol, at 200 K, C9H8O3". Zeitschrift für Kristallographie - New Crystal Structures. 229 (4): 331–332. doi:10.1515/ncrs-2014-0171. S2CID 93304005.
- ^ Thompson, Laurence K.; Mandal, Sanat K.; Tandon, Santokh S.; Bridson, John N.; Park, Murray K. (1996). "Magnetostructural Correlations in Bis(μ2-phenoxide)-Bridged Macrocyclic Dinuclear Copper(II) Complexes. Influence of Electron-Withdrawing Substituents on Exchange Coupling". Inorganic Chemistry. 35 (11): 3117–3125. doi:10.1021/IC9514197. PMID 11666507.
- ^ Gagne, R. R.; Spiro, C. L.; Smith, T. J.; Hamann, C. A.; Thies, W. R.; Shiemke, A. D. (1981). "The Synthesis, Redox Properties, and Ligand Binding of Heterobinuclear Transition-Metal Macrocyclic Ligand Complexes. Measurement of an Apparent Delocalization Energy in a Mixed-Valent CuICuII Complex". Journal of the American Chemical Society. 103 (14): 4073–4081. doi:10.1021/ja00404a017.
- ^ Lindoy, Leonard F. (July 1998). "Mono- and Diformylation of 4-Substituted Phenols: A New Application of the Duff Reaction". Synthesis. 1998 (7): 1029–1032. doi:10.1055/s-1998-2110.
- ^ Brühne, Friedrich; Wright, Elaine (2011). "Benzaldehyde". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_463.pub2. ISBN 978-3527306732.
