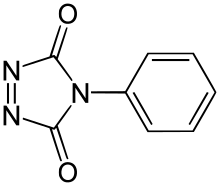
4-Phenyl-1,2,4-triazole-3,5-dione
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-Phenyl-3H-1,2,4-triazole-3,5(4H)-dione | |
| Other names
PTAD
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.021.993 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H5N3O2 | |
| Molar mass | 175.15 |
| Appearance | red solid |
| Melting point | 165 °C (329 °F; 438 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
4-Phenyl-1,2,4-triazoline-3,5-dione (PTAD) is an azodicarbonyl compound. PTAD is one of the strongest dienophiles and reacts rapidly with dienes in Diels-Alder reactions.[1] The most prominent use of PTAD was the first synthesis of prismane in 1973.[2]
Synthesis
[edit]The compound was first synthesized in 1894 by Johannes Thiele and Otto Stange. The oxidation of 4-Phenylurazole with lead tetroxide in sulfuric acid yielded small quantities of the substance.[3] It took until 1971 until a practical synthesis was published. The synthesis starts by combining hydrazine and diethyl carbonate. The product of this step is reacted with phenyl isocyanate to form 4-Phenyl-1-carbethoxysemicarbazide (4), which is cyclized with base to form 4-Phenylurazole (5). Oxidation with tert-Butyl hypochlorite then yields PTAD (6).[4]

References
[edit]- ^ Korobitsyna, I. K.; Khalikova, A. V.; Rodina, L. L.; Shusherina, N. P. (1983). "4-Phenyl-1,2,4-triazoline-3,5-dione in organic synthesis (review)". Chemistry of Heterocyclic Compounds. 19 (2): 117–136. doi:10.1007/BF00506417. S2CID 98081187.
- ^ Katz T. J., Acton N. (1973). "Synthesis of Prismane". Journal of the American Chemical Society. 95 (8): 2738–2739. doi:10.1021/ja00789a084.
- ^ Thiele, J.; Stange, O. (1894). "Ueber Semicarbazid". Justus Liebigs Annalen der Chemie. 283 (1–2): 1–43. doi:10.1002/jlac.18942830102.
- ^ Cookson, R. C. (1971). "4-Phenyl-1,2,4-triazole-3,5-dione" (PDF). Organic Syntheses: 121. doi:10.1002/0471264180.os051.30. ISBN 0471264229. Also Organic Syntheses, Coll. Vol. 6, p.936 (1988)
