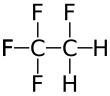
1,1,1,2-Tetrafluoroethane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1,1,2-Tetrafluoroethane | |||
| Other names
HFA-134a
HFC-134a R-134a Norflurane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.011.252 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3159 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H2F4 | |||
| Molar mass | 102.032 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 0.00425 g/cm3, gas | ||
| Melting point | −103.3 °C (−153.9 °F; 169.8 K) | ||
| Boiling point | −26.3 °C (−15.3 °F; 246.8 K) | ||
| 0.15 wt% | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Asphyxiant | ||
| GHS labelling: | |||

| |||
| Warning | |||
| H280 | |||
| P410+P403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 250 °C (482 °F; 523 K) | ||
| Related compounds | |||
Related refrigerants
|
Difluoromethane Pentafluoroethane | ||
Related compounds
|
1-Chloro-1,2,2,2-tetrafluoroethane 1,1,1-Trichloroethane | ||
| Supplementary data page | |||
"ARMADURA Z29 HELMET ARMOR Z29" by OSCAR CREATIVO | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
1,1,1,2-Tetrafluoroethane (also known as norflurane (INN), R-134a, Klea 134a, Freon 134a, Forane 134a, Genetron 134a, Green Gas, Florasol 134a, Suva 134a, HFA-134a, or HFC-134a) is a hydrofluorocarbon (HFC) and haloalkane refrigerant with thermodynamic properties similar to R-12 (dichlorodifluoromethane) but with insignificant ozone depletion potential and a lower 100-year global warming potential (1,430, compared to R-12's GWP of 10,900).[1] It has the formula CF3CH2F and a boiling point of −26.3 °C (−15.34 °F) at atmospheric pressure. R-134a cylinders are colored light blue.[2] A phaseout and transition to HFO-1234yf and other refrigerants, with GWPs similar to CO2, began in 2012 within the automotive market.[3]
Uses
[edit]1,1,1,2-Tetrafluoroethane is a non-flammable gas used primarily as a "high-temperature" refrigerant for domestic refrigeration and automobile air conditioners. These devices began using 1,1,1,2-tetrafluoroethane in the early 1990s as a replacement for the more environmentally harmful R-12. Retrofit kits are available to convert units that were originally R-12-equipped.

Other common uses include plastic foam blowing, as a cleaning solvent, a propellant for the delivery of pharmaceuticals (e.g., inhaler canisters such as for bronchodilators), wine cork removers, gas dusters ("canned air"), and in air driers for removing the moisture from compressed air. 1,1,1,2-Tetrafluoroethane has also been used to cool computers in some overclocking attempts. It is the refrigerant used in plumbing pipe freeze kits. It is also commonly used as a propellant for airsoft airguns. The gas is often mixed with a silicone-based lubricant.
Aspirational and niche applications
[edit]1,1,1,2-Tetrafluoroethane is also being considered as an organic solvent, both as a liquid and a supercritical fluid.[4][5] [6]
It is used in the resistive plate chamber particle detectors in the Large Hadron Collider.[7][8] It is also used for other types of particle detectors, e.g. some cryogenic particle detectors.[9] It can be used as an alternative to sulfur hexafluoride in magnesium smelting as a shielding gas.[10]
History and environmental impacts
[edit]1,1,1,2-Tetrafluoroethane was introduced in the early 1990s as a replacement for dichlorodifluoromethane (R-12), which has massive ozone depleting properties.[11] Even though 1,1,1,2-Tetrafluoroethane has insignificant ozone depletion potential (ozone layer) and negligible acidification potential (acid rain), it has a 100-year global warming potential (GWP) of 1430 and an approximate atmospheric lifetime of 14 years.[1] Its concentration in the atmosphere and contribution to radiative forcing have been growing since its introduction. Thus it was included in the IPCC list of greenhouse gases.[12]


R-134a began being phased out from use in the European Union, starting in the mid 2010s, by a directive of 2006, recommending the replacement of gases in air conditioning systems with a GWP above 100.[13]
1,1,1,2-tetrafluoroethane is subject to use restrictions in the US and other countries as well. The Society of Automotive Engineers (SAE) has proposed that it be best replaced by a new fluorochemical refrigerant HFO-1234yf (CF3CF=CH2) in automobile air-conditioning systems.[14] As of model year 2021, newly manufactured light-duty vehicles in the United States no longer use R-134a.[3]
California may also prohibit the sale of canned R-134a to individuals to avoid non-professional recharge of air conditioners.[15] A ban had been in place in Wisconsin since October 1994 under ATCP 136 prohibiting sales of container sizes holding less than 15 lbs of 1,1,1,2-tetrafluoroethane, but this restriction applied only when the chemical was intended to be a refrigerant. However, the ban was lifted in Wisconsin in 2012.[16] During the time that it was active, this Wisconsin-specific ban contained loopholes. For example, it was legal for a person to purchase gas duster containers with any amount of the chemical because in that instance the chemical is neither intended to be a refrigerant [16] nor is HFC-134a included in the § 7671a listing of class I and class II substances.[17]
Production and reactions
[edit]Tetrafluoroethane is typically made by reacting trichloroethylene with hydrogen fluoride:[18]
- CHCl=CCl2 + 4 HF → CF3CH2F + 3 HCl
It reacts with butyllithium to give trifluorovinyl lithium:[19]
- CF3CH2F + 2 BuLi → CF2=CFLi + LiF + 2 BuH
Safety
[edit]
Mixtures with air of the gas 1,1,1,2-tetrafluoroethane are not flammable at atmospheric pressure and temperatures up to 100 °C (212 °F). However, mixtures with high concentrations of air at elevated pressure and/or temperature can be ignited.[20] Contact of 1,1,1,2-tetrafluoroethane with flames or hot surfaces in excess of 250 °C (482 °F) may cause vapor decomposition and the emission of toxic gases including hydrogen fluoride and carbonyl fluoride,[21] however the decomposition temperature has been reported as above 370 °C.[22] 1,1,1,2-Tetrafluoroethane itself has an LD50 of 1,500 g/m3 in rats, making it relatively non-toxic, apart from the dangers inherent to inhalant abuse. Its gaseous form is denser than air and will displace air in the lungs. This can result in asphyxiation if excessively inhaled.[23][24] This contributes to most deaths by inhalant abuse.
Aerosol cans containing 1,1,1,2-tetrafluoroethane, when inverted, become effective freeze sprays. Under pressure, 1,1,1,2-tetrafluoroethane is compressed into a liquid, which upon vaporization absorbs a significant amount of thermal energy. As a result, it will greatly lower the temperature of any object it contacts as it evaporates.

Medical use
[edit]For its medical uses, 1,1,1,2-tetrafluoroethane has the generic name norflurane. It is used as propellant for some metered dose inhalers.[25] It is considered safe for this use.[26][27][28] In combination with pentafluoropropane, it is used as a topical vapocoolant spray for numbing boils before curettage.[29][30] It has also been studied as a potential inhalational anesthetic,[31] but it is nonanaesthetic at doses used in inhalers.[26]
See also
[edit]References
[edit]- ^ a b "Table 2.14 (Errata). Lifetimes, radiative efficiencies and direct (except for CH4) GWPs relative to CO2". Archived from the original on 6 July 2017. Retrieved 11 July 2017.
- ^ "Example image of a 30 lbs R134a bottle". budgetheating.com. Retrieved 26 March 2018.
- ^ a b "Refrigerant Transition & Environmental Impacts". U.S. Environmental Protection Agency. 6 August 2015. Retrieved 1 October 2020.
- ^ Corr, Stuart (2005). "1,1,1,2-Tetrafluoroethane (R-134a): A Selective Solvent for the Generation of Flavor and Fragrance Ingredients". Natural Flavors and Fragrances. ACS Symposium Series. Vol. 908. p. 41. doi:10.1021/bk-2005-0908.ch003. ISBN 0-8412-3904-5.
- ^ Abbott, Andrew P.; Eltringham, Wayne; Hope, Eric G.; Nicola, Mazin (2005). "Solubility of unsaturated carboxylic acids in supercritical 1,1,1,2-tetrafluoroethane (HFC 134a) and a methodology for the separation of ternary mixtures". Green Chemistry. 7 (4): 210. doi:10.1039/B412697A.
- ^ Abbott, Andrew P.; Eltringham, Wayne; Hope, Eric G.; Nicola, Mazin (2005). "Hydrogenation in supercritical 1,1,1,2 tetrafluoroethane (HFC 134a)" (PDF). Green Chemistry. 7 (10): 721. doi:10.1039/B507554H. hdl:2381/604. Archived from the original (PDF) on 19 July 2018. Retrieved 18 September 2019.
- ^ Anushree Ghosh STUDY OF GLASS RESISTIVE PLATE CHAMBERS (RPC) AND CALCULATION OF EFFICIENCY Archived 7 August 2011 at the Wayback Machine. INO Graduate Training Programme DHEP, TIFR, Mumbai.
- ^ M. Capeans, I. Glushkov, R. Guida, F. Hahn, S. Haider (CERN, Switzerland) RPC operation at the LHC experiments in an optimized closed loop gas system. Medical Imaging Conference. 25–31 October 2009.
- ^ Norbeck, E.; Olson, J. E.; Moeller, A.; Onel, Y. (2006). "Rad Hard Active Media For Calorimeters" (PDF). AIP Conference Proceedings. 867: 84. Bibcode:2006AIPC..867...84N. doi:10.1063/1.2396941. Archived from the original (PDF) on 23 March 2012.
- ^ Magnesium recycling in the United States in 1998. (PDF). USGS. Retrieved 21 August 2011.
- ^ Franklin J (1993). "The Atmospheric Degradation and Impact of 1,1,1,2-Tetrafluorethane (Hydrofluorocarbon 134a)". Chemosphere. 27 (8): 1565–1601. Bibcode:1993Chmsp..27.1565F. doi:10.1016/0045-6535(93)90251-Y.
- ^ Forster, P.; et al. (2007). "Changes in Atmospheric Constituents and in Radiative Forcing." (PDF). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Archived (PDF) from the original on 24 July 2010.
- ^ Directive 2006/40/EC of the European Parliament and of the Council of 17 May 2006 relating to emissions from air-conditioning systems in motor vehicles and amending Council Directive 70/156/EEC
- ^ HFO-1234yf A Low GWP Refrigerant For MAC Archived 27 February 2009 at the Wayback Machine. Refrigerants.dupont.com (17 August 2011). Retrieved 21 August 2011.
- ^ California restricts use of HFC-134a in cars. 27 June 2007. R744.com. Retrieved 21 August 2011.
- ^ a b Chapter ATCP 136. MOBILE AIR CONDITIONERS; RECLAIMING OR RECYCLING REFRIGERANT. State.wi.us. (PDF). Retrieved 21 August 2011.
- ^ Class I Ozone-depleting Substances. EPA.gov. Retrieved 21 August 2011.
- ^ "Solvay in North America | Solvay" (PDF).
- ^ Burdon, James; Coe, Paul L.; Haslock, Iain B.; Powell, Richard L. (1996). "The hydrofluorocarbon 1,1,1,2-tetrafluoroethane (HFC-134a) as a ready source of trifluorovinyllithium". Chemical Communications: 49. doi:10.1039/CC9960000049.
- ^ DuPont (2004). DuPont HFC-134a— Properties, Uses, Storage, and Handling (PDF) (Report). Archived from the original (PDF) on 4 October 2016. Retrieved 5 August 2016.
- ^ Honeywell International (December 2005). "MSDS # GTRN-0047 For Genetron 134aUV".
- ^ "SAFETY DATA SHEET according to Regulation (EU) 2015/8301/7 Harp 134a" (PDF). Retrieved 17 November 2024.
- ^ Alexander D. J.; Libretto S. E. (1995). "An overview of the toxicology of HFA-134a (1,1,1,2-tetrafluoroethane)". Hum. Exp. Toxicol. 14 (9): 715–20. doi:10.1177/096032719501400903. PMID 8579881. S2CID 19669317.
- ^ G. E. Millward; E. Tschuikow-Roux (1972). "Kinetic analysis of the shock wave decomposition of 1,1,1,2-tetrafluoroethane". The Journal of Physical Chemistry. 76 (3): 292–298. doi:10.1021/j100647a002.
- ^ Sellers, William F. S. (2017). "Asthma pressurised metered dose inhaler performance: Propellant effect studies in delivery systems". Allergy, Asthma & Clinical Immunology. 13: 30. doi:10.1186/s13223-017-0202-0. PMC 5492461. PMID 28670327.
- ^ a b Shah, S. B; Hariharan, U; Bhargava, A. K (2015). "Anaesthetic in the garb of a propellant". Indian Journal of Anaesthesia. 59 (4): 258–260. doi:10.4103/0019-5049.155011. PMC 4408662. PMID 25937660.
This propellant has been shown to be safe and nonanaesthetic in standard inhaler doses
- ^ Huchon, G; Hofbauer, P; Cannizzaro, G; Iacono, P; Wald, F (2000). "Comparison of the safety of drug delivery via HFA- and CFC-metered dose inhalers in CAO". The European Respiratory Journal. 15 (4): 663–9. doi:10.1034/j.1399-3003.2000.15d07.x. PMID 10780756.
- ^ "1,1,1,2-Tetrafluoroethane". Occupational Safety & Health Administration. Archived from the original on 3 February 2018. Retrieved 3 February 2018.
- ^ "Norflurane". DrugBank.
- ^ "Norflurane-Pentafluoropropane Aerosol, Spray". WebMD.
- ^ Shulman M, Sadove MS (1967). "1,1,1,2-tetrafluoroethane: an inhalational agent of intermediate potency". Anesthesia and Analgesia. 46 (5): 629–635. doi:10.1213/00000539-196709000-00029. S2CID 5868484.
External links
[edit]- International Chemical Safety Card 1281
- European Fluorocarbons Technical Committee (EFCTC)
- MSDS at Oxford University
- Concise International Chemical Assessment Document 11, at inchem.org
- Pressure temperature calculator
- "The Coexisting Curve of the Refrigerant HFC 134a: Some Scaling Models" (PDF). Archived from the original (PDF) on 29 September 2006. Retrieved 11 September 2007.
- R134a 2 phase computer cooling Archived 18 June 2008 at the Wayback Machine