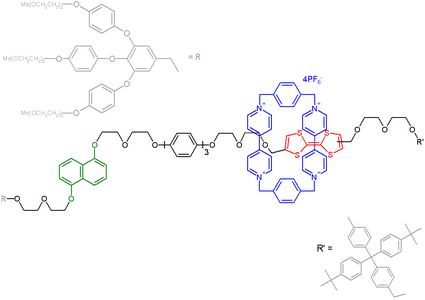
Molecular switch

A molecular switch is a molecule that can be switched between two or more stable or metastable states with the use of any external (exogenous) or internal (endogenous) stimuli, such as changes in pH, light, temperature, an electric current, a microenvironment, or in the presence of ions, and other ligands.[1][2] In some cases, a combination of stimuli is required[1]. Molecular switches are reversible. They have been considered for a wide area of possible applications, but the main uses are in photochromic lenses and windows.[1]
Biological
[edit]Biological stimuli are endogenous form of stimuli.[3] [4] This involves variation in the physiological changes around the cells, such as variable pH, presence of oxidative or reductive species, and enzymes.[5] In cellular biology, proteins act as intracellular signaling molecules by activating another protein in a signaling pathway. In order to do this, proteins act as molecular switches by toggling between active and inactive states[6].
For example, phosphorylation of proteins can be used to activate or inactivate proteins. The external signal flipping the molecular switch could be a protein kinase, which adds a phosphate group to the protein, or a protein phosphatase, which removes phosphate groups.[7] Normal tissues and diseased tissues have different pH, so current approaches of effective drug delivery systems (DDS) include the use of this difference in pH as an endogenous stimulus. Such DDS offer a huge advantage over the conventional therapeutic drug release methods as they selectively release drug cargo at a specific physiological pH.[8] For instance, a study by Shi et al. proposed a pH-responsive/enzyme-cascade-reactive nanoplatform for antibacterial applications.[9] Many artificial nucleic acid-based switches have opened up new opportunities in nucleic-acid nanoscience and RNA/DNA biochemistry.[1]
Acidochromic
[edit]The ability of some compounds to change color in function of the pH was known since the sixteenth century.[10] This effect was even known before the development of acid-base theory. Those are found in a wide range of plants like roses, cornflowers, primroses and violets. Robert Boyle was the first person to describe this effect, employing plant juices (in the forms of solution and impregnated paper).[11] This effect is the result of structural or electronic changes in molecules upon interaction with protons and is called acidochromism. Acidochromic molecular switches are most commonly used as pH indicators such as phenolphthalein, methyl orange, and methyl red. Their acidic and basic forms have different colors. When an acid or a base is added, the equilibrium between the two forms is displaced.[12]

Examples in the literature of molecular switches with reversible pH response are spiropyran,[13] hydrazones,[14] Donor-Acceptor-Steenhouse Aduucts (DASA),[15] heptamethine–oxonol dyes,[16] etc.
Spiropyran, SP changes its color from blue in the presence of acid such as TFA (trifluroacetic acid) to colorless ring opened form called merocyanine, MC while under alkaline conditions reverts it back to the ring closed, SP form.[17] They are called dual responsive switches since light can also be used to trigger the isomerization.[18] There mechanism of isomerization is shown in the figure above. Due to their easy synthesis and excellent optical stability, they are widely used in bioimaging and pH sensing.[19]
An interesting example of pH-responsive molecular switches is shown by Yin's group, who developed pH sensors made up of the spiropyran-based fluorescent probe that can be used for precise and rapid pH detection by making their pH paper strips. Their probe also incorporates indole salts as nucleophilic addition sites that react with OH- ions (hydroxide ions) in different pH environments.[20] A 2022 report by Wang et al. shows the spiropyran-based cellulose nanocrystals useful for pH sensors.[21]
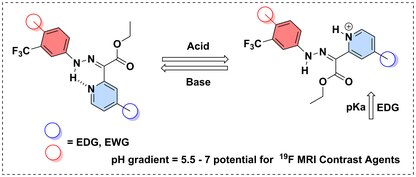
Acidochromic behavior of hydrazones (C=N-N-) is attributed to their tautomerization under an acidic or basic conditions.[22] This linkage is useful in drug delivery (DDS) due to their faster hydrolysis rate in an acidic environment.[23]
Acid can also help to tune the physical state of the switch. In 2022, Quintard and coworkers have shown the sol- gel transition of various amines using trichloroacetic acid (TCA) as fuel to create new types of time-controlled smart materials.[24]

Photochromic
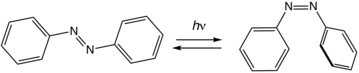
[edit]| Azobenzene | The photochromic trans-cis (E/Z) isomerization of azobenzenes has been used extensively in molecular switches. Upon isomerization, azobenzenes experience changes in physical properties, such as molecular geometry, absorption spectra, or dipole moment.[25][26]
Utilizing these changes allows azobenzenes to be used in various applications. In particular, azobenzenes incorporated into crown ethers give switchable receptors and azobenzenes in monolayers can provide light-controlled changes in surface properties.[27] |
|
| Diarylethenes | Diarylethenes undergo a fully reversible transformation between "ring-open" and "ring-closed" isomeric forms when exposed to light of different wavelengths.[28] Diarylethene-based photoswitches exhibit high photofatigue resistance, enabling them to undergo many photoswitching cycles with minimal degradation.[29] These compounds are also recognized in the development of long-lasting photochemical memory devices due to the thermal stability of both photoforms of diarylethenes.[28] | 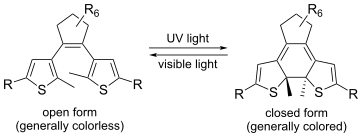
|
| Spiropyrans and spirooxazines | Spiropyrans, among the oldest photochromic compounds, are closely related to spirooxazines. After irradiation with UV light, the ring opens, forming a conjugated system with ability to absorb photons of visible light, and therefore appears colorful. When the UV source is removed, the molecules gradually relax to their ground state, the carbon-oxygen bond reforms, and the molecule returns to its colorless state. This class of photochromes, in particular, is thermodynamically unstable in one form and revert to the stable form in the dark unless cooled to low temperatures.[30][31][32] | 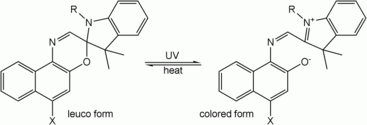
|
| Fulgides and Fulgimides | Similar to diarylethenes, the photochromic behavior of fulgides and fulgimides is based on 6π-electrocyclic ring-opening and ring-closing reactions.[33] They are highly photochromic photoswitches and reversibly interconvert between two isomeric forms when exposed to light of different wavelengths.[33][34] These compounds exhibit low photochemical fatigue, high thermal stability, as well as high conversion yields.[35][36] | 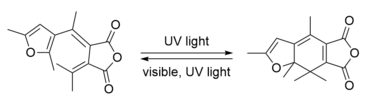
|
The molecules that isomerize when exposed to light of suitable wavelength are called photoswitches. Members of this class include azobenzenes, diarylethenes, dithienylethenes, fulgides, stilbenes, norbornadiene, spiropyrans, hydrazones, indigoids[37], diazocines,[38] and phenoxynaphthacene quinones.[39]
The inspiration to study light-sensitive switches came from an understanding of retinal. In dark, the 11 conjugated bonds exists in an all trans except cis configuration at C-11, which is cis. Retinal undergoes photoisomerization to the all-trans configuration.
Photo-induced structural, physical, or chemical changes can involve isomerization of bonds (cis <--> trans), electron transfer, proton transfer in the excited state, ring opening and closing mechanism.[40] These isomerizations affect optical properties. For example, the absorption maximum of (Z)-azobenzene is blue shifted with respect to (E)-azobenzene.[1]
Many light-driven azo-based switches have been investigated.[41]
Chiroptical
[edit]Chiroptical molecular switches are a specific subgroup with photochemical switching taking place between an enantiomers. In these compounds the "readout" is by circular dichroism.[42] Hindered alkenes can change their helicity (see: planar chirality) as response to irradiation with right or left-handed circularly polarized lightChiroptical molecular switches that show directional motion are considered synthetic molecular motors.[43] When attached to the end of a helical poly (isocyanate) polymer, they can switch the helical sense of the polymer.[44]
Redox active
[edit]Species that exist in more than oneredox state are potential switches. When the optical properties of the redox state differ, then redox is sometimes called electrochromism..[45] Ferrocene, which is orange, oxidizes to the blue ferrocenium cation.[46]
Many fluorescence based sensors are based on redox couple mechanism of switches which in their oxidized form quenches the fluorescence of fluorophore while in reduced state does not, or vice versa. Some other examples include, biindeno[2,1-b]thiophenylidene (BTP),[47] viologens, napthelene diimides,[48] bipyridinium,[49] and metal-ligand redox complex.[50]
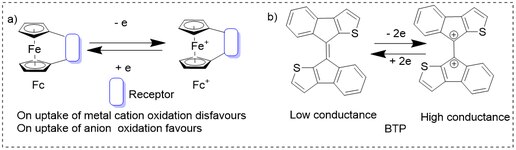
The first electrochemical sensors for selective binding of metal cations were designed using ferrocene.[51] Because of the high electrostatic repulsion metal cation (taken up by the receptor), the oxidation of ferrocene (Fc to Fc+) becomes difficult while this becomes more easier with anion uptake since it has negative charge to stabilize the system by attractive interactions, hence shifts the redox potential of Fc cathodically i.e., towards less positive direction. Thus, different redox properties of Fc help to do the selective binding of ions.[46] Further, biindeno[2,1-b]thiophenylidene on oxidation converts from the neutral to charged form which leads to the increase in the conductivity of the molecule and hence, they are used as actuators or dopants to modify the surface properties of polymers or nanomaterials.[47]

In 2024, Prof. Ben Feringa and his group reported a helicene featuring an indenofluorene-bridged bisthioxanthylidene as a novel switch that undergoes a two-electron redox process allowing it to modulate its (anti)aromatic character reversibly.[52]
Thermal Molecular switches
[edit]
In thermal molecular switches, conformational or structural change is induced by the temperature.[53] The mechanism responsible for thermochromic behavior is the gain of planarity in overcrowded alkenes, keto-enol tautomerization, a change in the crystalline structure (mainly found in inorganic materials such as change of octahedral crystal structure to tetrahedral),[54] the formation of free radicals, and ring-opening reactions.[55] Some molecules show reversible color change when they are heated or cooled respectively. Examples of thermochromic organic molecules include crowded ethenes (e.g., bianthrone and dixanthylene), schiff bases, and spiro compounds.
In 1999, the first example of thermochromic dye was published, in which 2,6-diphenyl-4-(2,4,6-triphenylpyridinio)phenolate (DTPP) and an indicator dye, Cresol Red embedded in a polymer gel network, are shown to exhibit an outstanding thermochromism.[56] The contrasting thermal response of RNA and DNA at variable temperatures is an interesting phenomenon. Tashiro et al. (2005) made a biomolecular device using this property of DNA and RNA.[57] They attached a fluorophore (2-aminopurene) to both DNA and RNA strands. The fluorescence signal of the fluorophore was turned "on" to "off" as the temperature changed from low to high for the DNA device and vice versa for the RNA device. Hence, successfully made reversible, thermoresponsive RNA- and DNA-based devices.

In 2011, Feng et al. reported a temperature-sensitive fluorescent triarylboron thermometer that shows high quantum yield and color change at a wide variety of temperatures.[58] Leuco dye (LD)-based thermochromic (TC) materials have been widely applied in energy storage, sensors, and optical memory storage.[59] A recent report in 2022 by Fei et al. demonstrated a four-input signal based optically controlled thermochromic switch. Azobenzene derivatives were used to lock the color developer and leuco dye at the required temperature.[60]
Supramolecular switches
[edit]Host-Guest
[edit]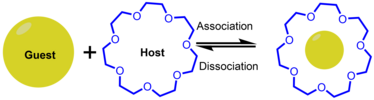
The first photochemical synthesis of crown ether via photochemical anthracene dimerization is described in 1978 by Desvergne & Bouas-Laurent.[61][62] Although not strictly speaking switchable, the compound is able to take up cations after a photochemical trigger; reverse was not possible with light. Solvent (acetonitrile) gives back the open form.
Anthracene groups serve as photo-antennae can control the conformations of crown ethers, which in turn control their chemical reactivity.[63] Upon absorption of light, they cab trigger trans-cis isomerization of the azo group, which resulted in ring expansion. Thus, in the trans orm the crown binds preferentially to ammonium, lithium, and sodium ions, while in the cis form the preference is for potassium and rubidium (both larger ions in the same alkali metal group). In the dark, the reverse isomerization takes place. This device concept mimics the biochemical action of monensin and nigericin: in a biphasic system, ions are taken up, triggered by light in one phase and deposited in the other phase in the absence of light.[64]
Apart from the solution phase modulation of interactions among host and guest molecules, solid phase interactions for their practical applications in functional devices have also been explored. Host materials embedded on nanomaterials showed better surface activity and sensing capabilities, enabling applications in nanotechnology, biology, environmental, and energy technologies.[65]
Mechanically-interlocked
[edit]
Some of the most advanced molecular switches are based on mechanically-interlocked molecular architectures where the bistable states differ in the position of the macrocycle.[66] These systems enable dynamic and reversible switching between different states in response to external stimuli like light, pH, redox processes, or mechanical force because they are made up of several molecular components that are spatially entangled but not covalently bound. Also, they provide better stability to the system by interlocking the guest molecules at the specific site, as compared to free unprotected guest in host guest molecular switches.
In 1991 Stoddart[67] devices a molecular shuttle based on a rotaxane on which a molecular bead is able to shuttle between two docking stations situated on a molecular thread. Stoddart predicts that when the stations are dissimilar with each of the stations addressed by a different external stimulus the shuttle becomes a molecular machine. In 1993, Stoddart is scooped by supramolecular chemistry pioneer Fritz Vögtle who actually delivers a switchable molecule based not on a rotaxane but on a related catenane.[68][69]
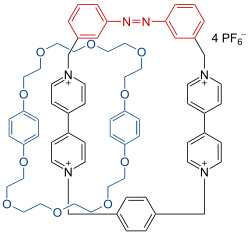
|
| |
| Photo switchable catenane Vögtle 1993 | Molecular switch Kaifer and Stoddart 1994 |
This compound is based on two ring systems: one ring holds the photoswichable azobenzene ring and two paraquat docking stations and the other ring is a polyether with to arene rings with binding affinity for the paraquat units. In this system NMR spectroscopy shows that in the azo trans-form the polyether ring is free to rotate around its partner ring but then when a light trigger activates the cis azo form this rotation mode is stopped.
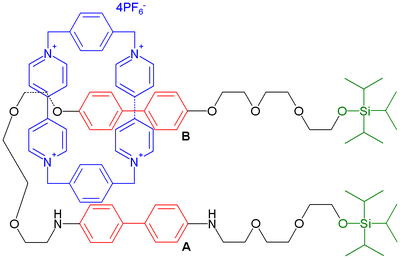
Molecular shuttles were utilized in an experimental DRAM circuit.[70] The device consists of 400 bottom silicon nanowire electrodes (16 nanometer (nm) wide at 33 nm intervals) crossed by another 400 titanium top-nanowires with similar dimensions sandwiching a monolayer of a bistable rotaxane depicted below:
The hydrophilic diethylene glycol stopper on the left (gray) is specifically designed to anchor to the silicon wire (made hydrophilic by phosphorus doping) while the hydrophobic tetraarylmethane stopper on the right does the same to the likewise hydrophobic titanium wire. In the ground state of the switch, the paraquat ring is located around a tetrathiafulvalene unit (in red) but it moves to the dioxynaphthyl unit (in green) when the fulvalene unit is oxidized by application of a current. When the fulvalene is reduced back a metastable high conductance '1' state is formed which relaxes back to the ground state with a chemical half-life of around one hour. The problem of defects is circumvented by adopting a defect-tolerant architecture also found in the Teramac project. In this way a circuit is obtained consisting of 160,000 bits on an area the size of a white blood cell translating into 1011 bits per square centimeter.
References
[edit]- ^ a b c d e Feringa BL, Browne WR, eds. (July 2011). Molecular Switches (1st ed.). Wiley. doi:10.1002/9783527634408. ISBN 978-3-527-31365-5.
- ^ Amendola V, Bonizzoni M, Fabbrizzi L (July 2011), Feringa BL, Browne WR (eds.), "Ion Translocation within Multisite Receptors", Molecular Switches, Wiley, pp. 361–398, doi:10.1002/9783527634408.ch11, ISBN 978-3-527-31365-5, retrieved 2025-02-21
- ^ Viricel W, Mbarek A, Leblond J (October 2015). "Switchable Lipids: Conformational Change for Fast pH-Triggered Cytoplasmic Delivery" (PDF). Angewandte Chemie. 54 (43): 12743–12747. doi:10.1002/anie.201504661. PMID 26189870. S2CID 24175578.
- ^ Raza A, Rasheed T, Nabeel F, Hayat U, Bilal M, Iqbal HM (March 2019). "Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release". Molecules. 24 (6): 1117. doi:10.3390/molecules24061117. PMC 6470858. PMID 30901827.
- ^ Kang H, Yang Y, Wei B (May 2024). "Synthetic molecular switches driven by DNA-modifying enzymes". Nature Communications. 15 (1): 3781. Bibcode:2024NatCo..15.3781K. doi:10.1038/s41467-024-47742-2. PMC 11074287. PMID 38710688.
- ^ Alberts B, Johnson AD, Lewis J, Morgan D, Raff MC, Roberts K, et al. (2015). Molecular Biology of the Cell (6th ed.). London: Garland Science, Taylor & Francis Group. ISBN 9780815344322. OCLC 1004752557.
- ^ Ardito F, Giuliani M, Perrone D, Troiano G, Lo Muzio L (August 2017). "The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review)". International Journal of Molecular Medicine. 40 (2): 271–280. doi:10.3892/ijmm.2017.3036. PMC 5500920. PMID 28656226.
- ^ Zhang Y, Zhu J, Qiu L, Lv Z, Zhao Z, Ren X, et al. (February 2025). "Stimulus-activated ribonuclease targeting chimeras for tumor microenvironment activated cancer therapy". Nature Communications. 16 (1): 1288. Bibcode:2025NatCo..16.1288Z. doi:10.1038/s41467-025-56691-3. PMC 11790973. PMID 39900602.
- ^ Shi Y, Cao Y, Cheng J, Yu W, Liu M, Yin J, et al. (February 2022). "Construction of Self-Activated Nanoreactors for Cascade Catalytic Anti-Biofilm Therapy Based on H2O2 Self-Generation and Switch-On NO Release". Advanced Functional Materials. 32 (20): 2111148. doi:10.1002/adfm.202111148. ISSN 1616-3028.
- ^ Szabadvary F, Oesper RE (May 1964). "Indicators: A historical perspective". Journal of Chemical Education. 41 (5): 285. Bibcode:1964JChEd..41..285S. doi:10.1021/ed041p285.
- ^ Okuyama T, Maskill H (November 2013). "Acids and Bases". Organic Chemistry: A Mechanistic Approach. Oxford University Press. doi:10.1093/hesc/9780199693276.003.0006. ISBN 978-0-19-969327-6. Retrieved 2025-02-23.
- ^ Helmenstine AM. "pH indicator definition and examples". ThoughtCo.
- ^ Kortekaas L, Browne WR (June 2019). "The evolution of spiropyran: fundamentals and progress of an extraordinarily versatile photochrome". Chemical Society Reviews. 48 (12): 3406–3424. doi:10.1039/C9CS00203K. PMID 31150035.
- ^ Shao B, Aprahamian I (September 2020). "Hydrazones as New Molecular Tools". Chem. 6 (9): 2162–2173. Bibcode:2020Chem....6.2162S. doi:10.1016/j.chempr.2020.08.007.
- ^ Fiorentino A, Sachini B, Corra S, Credi A, Femoni C, Fraix A, et al. (October 2022). "Acidochromism of donor-acceptor Stenhouse adducts in organic solvent". Chemical Communications. 58 (80): 11236–11239. doi:10.1039/D2CC03761K. PMID 35968687.
- ^ Mourot B, Mazan V, Elhabiri M, Sarkar R, Jacquemin D, Siri O, et al. (January 2024). "Insights into extended coupled polymethines through the investigation of dual UV-to-NIR acidochromic switches based on heptamethine-oxonol dyes". Chemical Science. 15 (4): 1248–1259. doi:10.1039/D3SC06126D. PMC 10806682. PMID 38274067.
- ^ Wojtyk JT, Wasey A, Xiao NN, Kazmaier PM, Hoz S, Yu C, et al. (April 2007). "Elucidating the mechanisms of acidochromic spiropyran-merocyanine interconversion". The Journal of Physical Chemistry. A. 111 (13): 2511–2516. Bibcode:2007JPCA..111.2511W. doi:10.1021/jp068575r. PMID 17388360.
- ^ Razavi B, Abdollahi A, Roghani-Mamaqani H, Salami-Kalajahi M (April 2020). "Light-, temperature-, and pH-responsive micellar assemblies of spiropyran-initiated amphiphilic block copolymers: Kinetics of photochromism, responsiveness, and smart drug delivery". Materials Science & Engineering. C, Materials for Biological Applications. 109: 110524. doi:10.1016/j.msec.2019.110524. PMID 32228960.
- ^ Viricel W, Mbarek A, Leblond J (October 2015). "Switchable Lipids: Conformational Change for Fast pH-Triggered Cytoplasmic Delivery" (PDF). Angewandte Chemie. 54 (43): 12743–12747. doi:10.1002/anie.201504661. PMID 26189870.
- ^ Ren H, Huo F, Zhang Y, Zhao S, Yin C (September 2020). "An NIR ESIPT-based fluorescent probe with large stokes shift for specific detection of Cys and its bioimaging in cells and mice". Sensors and Actuators B: Chemical. 319: 128248. Bibcode:2020SeAcB.31928248R. doi:10.1016/j.snb.2020.128248.
- ^ Wang J, Avram L, Diskin-Posner Y, Białek MJ, Stawski W, Feller M, et al. (November 2022). "Altering the Properties of Spiropyran Switches Using Coordination Cages with Different Symmetries". Journal of the American Chemical Society. 144 (46): 21244–21254. Bibcode:2022JAChS.14421244W. doi:10.1021/jacs.2c08901. PMC 9706567. PMID 36377832.
- ^ Landge SM, Aprahamian I (December 2009). "A pH activated configurational rotary switch: controlling the E/Z isomerization in hydrazones". Journal of the American Chemical Society. 131 (51): 18269–18271. Bibcode:2009JAChS.13118269L. doi:10.1021/ja909149z. PMID 19968272.
- ^ Sonawane SJ, Kalhapure RS, Govender T (March 2017). "Hydrazone linkages in pH responsive drug delivery systems". European Journal of Pharmaceutical Sciences. 99: 45–65. doi:10.1016/j.ejps.2016.12.011. PMID 27979586.
- ^ a b Olivieri E, Gasch B, Quintard G, Naubron JV, Quintard A (June 2022). "Dissipative Acid-Fueled Reprogrammable Supramolecular Materials" (PDF). ACS Applied Materials & Interfaces. 14 (21): 24720–24728. doi:10.1021/acsami.2c01608. PMID 35580903.
- ^ Beharry AA, Woolley GA (2011). "Azobenzene photoswitches for biomolecules". Chemical Society Reviews. 40 (8): 4422. doi:10.1039/c1cs15023e. ISSN 0306-0012.
- ^ Merino E, Ribagorda M (2012). "Control over molecular motion using the cis – trans photoisomerization of the azo group". Beilstein Journal of Organic Chemistry. 8: 1071–1090. doi:10.3762/bjoc.8.119. ISSN 1860-5397. PMC 3458724. PMID 23019434.
- ^ Shiga M, Takagi M, Ueno K (1980). "AZO-CROWN ETHERS. THE DYES WITH AZO GROUP DIRECTLY INVOLVED IN THE CROWN ETHER SKELETON". Chemistry Letters. 9 (8): 1021–1022. doi:10.1246/cl.1980.1021. ISSN 0366-7022.
- ^ a b Irie M, Mohri M (1988). "Thermally irreversible photochromic systems. Reversible photocyclization of diarylethene derivatives". The Journal of Organic Chemistry. 53 (4): 803–808. doi:10.1021/jo00239a022. ISSN 0022-3263.
- ^ Irie M, Fukaminato T, Matsuda K, Kobatake S (2014). "Photochromism of Diarylethene Molecules and Crystals: Memories, Switches, and Actuators". Chemical Reviews. 114 (24): 12174–12277. doi:10.1021/cr500249p. ISSN 0009-2665.
- ^ Baillet G, Giusti G, Guglielmetti R (1993). "Comparative photodegradation study between spiro[indoline—oxazine] and spiro[indoline—pyran] derivatives in solution". Journal of Photochemistry and Photobiology A: Chemistry. 70 (2): 157–161. doi:10.1016/1010-6030(93)85036-8.
- ^ Ballet G (1997). "Photodegradation of Organic Photochromes in Polymers - Naphthopyrans and Naphthoxazines Series -". Molecular Crystals and Liquid Crystals Science and Technology. Section A. Molecular Crystals and Liquid Crystals. 298 (1): 75–82. doi:10.1080/10587259708036145. ISSN 1058-725X.
- ^ Baillet G, Giusti G, Guglielmetti R (1995). "Study of the Fatigue Process and the Yellowing of Polymeric Films Containing Spirooxazine Photochromic Compounds". Bulletin of the Chemical Society of Japan. 68 (4): 1220–1225. doi:10.1246/bcsj.68.1220. ISSN 0009-2673.
- ^ a b Renth F, Siewertsen R, Temps F (2013). "Enhanced photoswitching and ultrafast dynamics in structurally modified photochromic fulgides". International Reviews in Physical Chemistry. 32 (1): 1–38. doi:10.1080/0144235X.2012.729331. ISSN 0144-235X.
- ^ Browne WR, Feringa BL (2011). "Chiroptical Molecular Switches". Molecular Switches: 121–179. doi:10.1002/9783527634408.ch5.
- ^ Koshima H, Nakaya H, Uchimoto H, Ojima N (2012). "Photomechanical Motion of Furylfulgide Crystals". Chemistry Letters. 41 (1): 107–109. doi:10.1246/cl.2012.107. ISSN 0366-7022.
- ^ Harada J, Taira M, Ogawa K (2017). "Photochromism of Fulgide Crystals: From Lattice-Controlled Product Accumulation to Phase Separation". Crystal Growth & Design. 17 (5): 2682–2687. doi:10.1021/acs.cgd.7b00182. ISSN 1528-7483.
- ^ Fischer T, Leitner J, Gerwien A, Mayer P, Dreuw A, Dube H, et al. (July 2023). "Mechanistic Elucidation of the Hula-Twist Photoreaction in Hemithioindigo". Journal of the American Chemical Society. 145 (27): 14811–14822. Bibcode:2023JAChS.14514811F. doi:10.1021/jacs.3c03536. ISSN 0002-7863. PMC 10347542. PMID 37364887.
- ^ Lentes P, Frühwirt P, Freißmuth H, Moormann W, Kruse F, Gescheidt G, et al. (March 2021). "Photoswitching of Diazocines in Aqueous Media". The Journal of Organic Chemistry. 86 (5): 4355–4360. doi:10.1021/acs.joc.1c00065. ISSN 0022-3263. PMID 33606536.
- ^ Buchholtz F, Zelichenok A, Krongauz V (March 1993). "Synthesis of new photochromic polymers based on phenoxynaphthacenequinone". Macromolecules. 26 (5): 906–910. Bibcode:1993MaMol..26..906B. doi:10.1021/ma00057a004. ISSN 0024-9297.
- ^ Dinda B (November 2017), "Principles of Photochemical Reactions", Essentials of Pericyclic and Photochemical Reactions, Lecture Notes in Chemistry, vol. 93, Cham: Springer International Publishing, pp. 181–214, doi:10.1007/978-3-319-45934-9_6, ISBN 978-3-319-45934-9, retrieved 2025-02-25
- ^ Wang Z, Hölzel H, Moth-Poulsen K (June 2022). "Status and challenges for molecular solar thermal energy storage system based devices". Chemical Society Reviews. 51 (17): 7313–7326. Bibcode:2022ChSRv..51.7313W. doi:10.1039/D1CS00890K. ISSN 0306-0012. PMC 9426646. PMID 35726574.
- ^ Mammana A, Carroll GT, Feringa BL (February 2012). "Circular dichroism of dynamic systems: switching molecular and supramolecular chirality.". Comprehensive Chiroptical spectroscopy: applications in Stereochemical analysis of synthetic compounds, natural products, and biomolecules. Vol. 2. pp. 289–316. doi:10.1002/9781118120392.ch8. ISBN 978-1-118-01292-5.
- ^ Feringa BL, van Delden RA, Koumura N, Geertsema EM (May 2000). "Chiroptical Molecular Switches" (PDF). Chemical Reviews. 100 (5): 1789–1816. doi:10.1021/cr9900228. PMID 11777421.
- ^ Carroll GT, Jongejan MG, Pijper D, Feringa BL (July 2010). "Spontaneous generation and patterning of chiral polymeric surface toroids". Chemical Science. 1 (4): 469. doi:10.1039/c0sc00159g. ISSN 2041-6520. S2CID 96957407.
- ^ Gu C, Jia AB, Zhang YM, Zhang SX (September 2022). "Emerging Electrochromic Materials and Devices for Future Displays". Chemical Reviews. 122 (18): 14679–14721. doi:10.1021/acs.chemrev.1c01055. ISSN 0009-2665. PMC 9523732. PMID 35980039.
- ^ a b Fabbrizzi L (December 2020). "The ferrocenium/ferrocene couple: a versatile redox switch". ChemTexts. 6 (4): 22. Bibcode:2020ChTxt...6...22F. doi:10.1007/s40828-020-00119-6. ISSN 2199-3793.
- ^ a b Ohtake T, Tanaka H, Matsumoto T, Kimura M, Ohta A (July 2014). "Redox-Driven Molecular Switches Consisting of Bis(benzodithiolyl)bithienyl Scaffold and Mesogenic Moieties: Synthesis and Complexes with Liquid Crystalline Polymer". The Journal of Organic Chemistry. 79 (14): 6590–6602. doi:10.1021/jo501072u. ISSN 0022-3263. PMID 24955756.
- ^ Medabalmi V, Sundararajan M, Singh V, Baik MH, Byon HR (May 2020). "Naphthalene diimide as a two-electron anolyte for aqueous and neutral pH redox flow batteries". Journal of Materials Chemistry A. 8 (22): 11218–11223. doi:10.1039/D0TA01160F. ISSN 2050-7488.
- ^ Han Y, Nickle C, Zhang Z, Astier HP, Duffin TJ, Qi D, et al. (August 2020). "Electric-field-driven dual-functional molecular switches in tunnel junctions". Nature Materials. 19 (8): 843–848. Bibcode:2020NatMa..19..843H. doi:10.1038/s41563-020-0697-5. ISSN 1476-1122.
- ^ Kalny D, Elhabiri M, Moav T, Vaskevich A, Rubinstein I, Shanzer A, et al. (June 2002). "A new molecular switch: redox-driven translocation mechanism of the copper cation". Chemical Communications (13): 1426–1427. doi:10.1039/b204145f.
- ^ Dietrich B, Lehn J, Sauvage J (January 1969). "Les Cryptates". Tetrahedron Letters (in French). 10 (34): 2889–2892. doi:10.1016/S0040-4039(01)88300-3.
- ^ Sidler E, Hein R, Doellerer D, Feringa BL (July 2024). "Redox-Switchable Aromaticity in a Helically Extended Indeno[2,1- c ]fluorene". Journal of the American Chemical Society. 146 (28): 19168–19176. Bibcode:2024JAChS.14619168S. doi:10.1021/jacs.4c04191. ISSN 0002-7863. PMC 11258684. PMID 38954739.
- ^ Day JH (January 1963). "Thermochromism". Chemical Reviews. 63 (1): 65–80. doi:10.1021/cr60221a005. ISSN 0009-2665.
- ^ Day JH (December 1968). "Thermochromism of inorganic compounds". Chemical Reviews. 68 (6): 649–657. doi:10.1021/cr60256a001. ISSN 0009-2665.
- ^ Seeboth A, Lötzsch D, eds. (December 2013). Thermochromic and Thermotropic Materials (0 ed.). Jenny Stanford Publishing. doi:10.1201/b16299. ISBN 978-0-429-07428-8.
- ^ Seeboth A, Kriwanek J, Vetter R (1999). "The first example of thermochromism of dyes embedded in transparent polymer gel networks". Journal of Materials Chemistry. 9 (10): 2277–2278. doi:10.1039/a906159b.
- ^ Tashiro R, Sugiyama H (February 2005). "Biomolecule-Based Switching Devices that Respond Inversely to Thermal Stimuli". Journal of the American Chemical Society. 127 (7): 2094–2097. Bibcode:2005JAChS.127.2094T. doi:10.1021/ja044138j. ISSN 0002-7863. PMID 15713085.
- ^ Feng J, Tian K, Hu D, Wang S, Li S, Zeng Y, et al. (August 2011). "A Triarylboron-Based Fluorescent Thermometer: Sensitive Over a Wide Temperature Range". Angewandte Chemie International Edition. 50 (35): 8072–8076. doi:10.1002/anie.201102390. ISSN 1433-7851. PMID 21739545.
- ^ Muthyala R (April 2006). Chemistry and Applications of Leuco Dyes. Topics in Applied Chemistry. Boston, MA: Kluwer Academic Publishers. ISBN 978-0-306-45459-2.
- ^ Fei L, Yu W, Wu Z, Yin Y, Moth Poulsen K, Wang C (November 2022). "Optically Controlled Thermochromic Switching for Multi-Input Molecular Logic". Angewandte Chemie International Edition. 61 (44): e202212483. doi:10.1002/anie.202212483. ISSN 1433-7851. PMID 36102669.
- ^ Bouas-Laurent H, Castellan A, Desvergne JP (January 1980). "From anthracene photodimerization to jaw photochromic materials and photocrowns". Pure and Applied Chemistry. 52 (12): 2633–2648. doi:10.1351/pac198052122633. ISSN 1365-3075.
- ^ Desvergne JP, Bouas-Laurent H (1978). "Cation complexing photochromic materials involving bisanthracenes linked by a polyether chain. Preparation of a crown-ether by photocycloisomerization". Journal of the Chemical Society, Chemical Communications (9): 403. doi:10.1039/c39780000403. ISSN 0022-4936.
- ^ Shinkai S, Nakaji T, Nishida Y, Ogawa T, Manabe O (August 1980). "Photoresponsive crown ethers. 1. Cis-trans isomerism of azobenzene as a tool to enforce conformational changes of crown ethers and polymers". Journal of the American Chemical Society. 102 (18): 5860–5865. Bibcode:1980JAChS.102.5860S. doi:10.1021/ja00538a026. ISSN 0002-7863.
- ^ Shinkai S, Nakaji T, Ogawa T, Shigematsu K, Manabe O (January 1981). "Photoresponsive crown ethers. 2. Photocontrol of ion extraction and ion transport by a bis(crown ether) with a butterfly-like motion". Journal of the American Chemical Society. 103 (1): 111–115. Bibcode:1981JAChS.103..111S. doi:10.1021/ja00391a021. ISSN 0002-7863.
- ^ Yang YW, Sun YL, Song N (July 2014). "Switchable Host–Guest Systems on Surfaces". Accounts of Chemical Research. 47 (7): 1950–1960. doi:10.1021/ar500022f. ISSN 0001-4842. PMID 24635353.
- ^ Stoddart JF (May 2009). "The chemistry of the mechanical bond". Chemical Society Reviews. 38 (6): 1802. doi:10.1039/b819333a. ISSN 0306-0012.
- ^ Anelli PL, Spencer N, Stoddart JF (June 1991). "A molecular shuttle". Journal of the American Chemical Society. 113 (13): 5131–5133. Bibcode:1991JAChS.113.5131A. doi:10.1021/ja00013a096. PMID 27715028.
- ^ Vögtle F, Müller WM, Müller U, Bauer M, Rissanen K (September 1993). "Photoswitchable catenanes". Angewandte Chemie International Edition in English. 32 (9): 1295–1297. doi:10.1002/anie.199312951.
- ^ Benniston AC, Harriman A (October 1993). "A Light-Induced Molecular Shuttle Based on a [2] Rotaxane-Derived Triad". Angewandte Chemie International Edition in English. 32 (10): 1459–1461. doi:10.1002/anie.199314591.
- ^ Green JE, Choi JW, Boukai A, Bunimovich Y, Johnston-Halperin E, Delonno E, et al. (January 2007). "A 160-kilobit molecular electronic memory patterned at 10^(11) bits per square centimetre". Nature. 445 (7126): 414–417. doi:10.1038/nature05462. PMID 17251976.
Further reading
[edit]- Feringa BL, Browne WR (2011-08-04). Molecular Switches (2nd ed.). Weinheim, Germany: Wiley-VCH. ISBN 9783527634415. OCLC 1132137894.