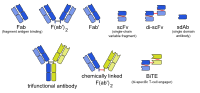
Bi-specific T-cell engager

Bi-specific T-cell engager (BiTE) is a class of artificial bispecific monoclonal antibodies that are investigated for use as anti-cancer drugs. They direct a host's immune system, more specifically the T cells' cytotoxic activity, against cancer cells. BiTE is a registered trademark of Micromet AG (fully owned subsidiary of Amgen Inc).[1]
BiTE molecules are fusion proteins consisting of two single-chain variable fragments (scFvs) of different antibodies, or amino acid sequences from four different genes, on a single peptide chain of about 55 kilodaltons. One of the scFvs binds to T cells via the CD3 receptor, and the other to a tumor cell via a tumor specific molecule.[2][3]
Mechanism of action
[edit]
Like other bispecific antibodies, and unlike ordinary monoclonal antibodies, BiTEs form a link between T cells and tumor cells. This causes T cells to exert cytotoxic activity on tumor cells by producing proteins like perforin and granzymes, independently of the presence of MHC I or co-stimulatory molecules. These proteins enter tumor cells and initiate the cell's apoptosis.[2][4]
This action mimics physiological processes observed during T cell attacks against tumor cells.[4]
BiTEs in clinical assessment or with clinical approvals
[edit]Several BiTEs are currently in preclinical and clinical trials to assess their therapeutic efficacy and safety. [5]
Blinatumomab
[edit]Blinatumomab links T cells with CD19 receptors found on the surface of B cells. The Food and Drug Administration (US) and the European Medicines Agency approved this therapy for adults with Philadelphia chromosome-negative relapsed or refractory acute lymphoblastic leukemia.[6]
Glofitamab
[edit]It is a bispecific CD20-directed CD3 T-cell engager. It was approved for medical use in Canada in March 2023, in the United States in June 2023, and in the European Union in July 2023.
Mosunetuzumab
[edit]Bispecifically binds CD20 and CD3 to engage T-cells. Mosunetuzumab was approved for medical use in the European Union in June 2022.
Solitomab
[edit]Solitomab links T cells with the EpCAM antigen which is expressed by colon, gastric, prostate, ovarian, lung, and pancreatic cancers.[7][8]
Talquetamab
[edit]
Talquetamab, sold under the brand name Talvey, is a humanized monoclonal antibody used for the treatment of multiple myeloma.[9][10] It is a bispecific GPRC5D-directed CD3 T-cell engager.[9] Talquetamab is a bispecific antibody against two targets: human CD3, a T-cell surface antigen, and human G-protein coupled receptor family C group 5 member D (GPRC5D), a tumor-associated antigen with potential antineoplastic activity.[11] Talquetamab binds both targets, drawing the T cells close to the tumor cells, causing a cytotoxic T-lymphocyte response.[11] It is being developed by Janssen Pharmaceuticals.[12]
The most common adverse reactions include cytokine release syndrome, dysgeusia, nail disorder, musculoskeletal pain, skin disorder, rash, fatigue, decreased weight, dry mouth, pyrexia, xerosis, dysphagia, upper respiratory tract infection, and diarrhea.[13]
Talquetamab was approved for medical use in both the United States[9][13][14] and the European Union[15] in August 2023. The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[16]Tarlatamab
[edit]
Tarlatamab, sold under the brand name Imdelltra, is an anti-cancer medication used for the treatment of extensive-stage small cell lung cancer.[17] It is a bispecific T-cell engager that binds delta-like ligand 3 and CD3.[17]
The most common adverse reactions include cytokine release syndrome, fatigue, pyrexia, dysgeusia, decreased appetite, musculoskeletal pain, and constipation, anemia and nausea.[18]
It was approved for medical use in the United States in May 2024.[18][19]Tebentafusp
[edit]After clinical trials, in January 2022, the US FDA approved tebentafusp (a BiTE targeting the gp100 peptide) for HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma.[20]
Epcoritamab
[edit]Epcoritamab, sold under the brand name Epkinly, is used for the treatment of diffuse large B-cell lymphoma. Epcoritamab is a bispecific CD20-directed CD3 T-cell engager.
Epcoritamab was approved for medical use in the United States in May 2023,[21][22][23][24][25] in the European Union in September 2023, and in Canada in December 2023.
Further research
[edit]Utilizing the same technology, melanoma (with MCSP specific BiTEs) and acute myeloid leukemia (with CD33 specific BiTEs) can be targeted.[26] As of 2008[update], research in this area is active.[26]
Another avenue for novel anti-cancer therapies is re-engineering some of the currently used conventional antibodies like trastuzumab (targeting HER2/neu), cetuximab and panitumumab (both targeting the EGF receptor), using the BiTE approach.[27]
As of 2009[update], BiTEs against CD66e and EphA2 are being developed as well.[28]
References
[edit]- ^ "US Trademark registration no. 3,068,856, serial number 78/040,636". US Patent and Trademark Office.
- ^ a b Helwick, Caroline (1 June 2008). "Novel BiTE antibody mediates contact between T cells and cancer cells". Oncology NEWS International. 17 (6). Archived from the original on 16 February 2012. Retrieved 15 August 2008.
- ^ Rüttinger, D.; Zugmaier, G.; Nagorsen, D.; Reinhardt, C.; Baeuerle, P. A. (2008). "BiTE-Antikörper: Durch Bispezifität T-Lymphozyten gegen Tumorzellen richten" [BiTE antibodies: Directing T lymphocytes against tumor cells by bispecifity]. Journal Onkologie (in German) (4).
- ^ a b "BiTE Antibody Platform". Micromet Inc.
- ^ Voynov, V; Adam, PJ (2020). "Discovery Strategies to Maximize the Clinical Potential of T-Cell Engaging Antibodies for the Treatment of Solid Tumors". Antibodies. 9 (4): E65–E81. doi:10.3390/antib9040065. PMC 7709135. PMID 33217946. S2CID 227100306.
- ^ Malard, Florent; Mohty, Mohamad (4 April 2020). "Acute lymphoblastic leukaemia". The Lancet. 395 (10230): 1146–1162. doi:10.1016/s0140-6736(19)33018-1. ISSN 0140-6736. PMID 32247396. S2CID 214779717.
- ^ Amann, M.; d'Argouges, S.; Lorenczewski, G.; Brischwein, K.; Kischel, R.; Lutterbuese, R.; Mangold, S.; Rau, D.; Volkland, J.; Pflanz, S.; Raum, T.; Münz, M.; Kufer, P.; Schlereth, B.; Baeuerle, P. A.; Friedrich, M. (2009). "Antitumor Activity of an EpCAM/CD3-bispecific BiTE Antibody During Long-term Treatment of Mice in the Absence of T-cell Anergy and Sustained Cytokine Release". Journal of Immunotherapy. 32 (5): 452–464. doi:10.1097/CJI.0b013e3181a1c097. PMID 19609237. S2CID 25568468.
- ^ Kebenko, Maxim; Goebeler, Marie-Elisabeth; Wolf, Martin; Hasenburg, Annette; Seggewiss-Bernhardt, Ruth; Ritter, Barbara; Rautenberg, Beate; Atanackovic, Djordje; Kratzer, Andrea; Rottman, James B.; Friedrich, Matthias (2018). "A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors". Oncoimmunology. 7 (8): e1450710. doi:10.1080/2162402X.2018.1450710. ISSN 2162-4011. PMC 6136859. PMID 30221040.
- ^ a b c "Talvey- talquetamab injection". DailyMed. U.S. National Library of Medicine. 18 August 2023. Archived from the original on 24 August 2023. Retrieved 23 August 2023.
- ^ "Talvey EPAR". European Medicines Agency. 21 September 2023. Retrieved 6 October 2023.
- ^ a b "Talquetamab". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 11 August 2023. Retrieved 30 January 2023.
- ^ Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk NW, Rodríguez-Otero P, Askari E, Mateos MV, Costa LJ, Caers J, Verona R, Girgis S, Yang S, Goldsmith RB, Yao X, Pillarisetti K, Hilder BW, Russell J, Goldberg JD, Krishnan A (December 2022). "Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma". The New England Journal of Medicine. 387 (24): 2232–2244. doi:10.1056/NEJMoa2204591. PMID 36507686. S2CID 254560960.
- ^ a b "FDA grants accelerated approval to talquetamab-tgvs for relapsed or refractory multiple myeloma". U.S. Food and Drug Administration (FDA). 9 August 2023. Archived from the original on 11 August 2023. Retrieved 10 August 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Theoret MR (2023). "Talvey (talquetamab-tgvs) injection" (PDF). Approval Letter. U.S. Food and Drug Administration. Archived from the original (PDF) on 11 August 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Talvey Product information". Union Register of medicinal products. 22 August 2023. Archived from the original on 25 August 2023. Retrieved 25 August 2023.
- ^ New Drug Therapy Approvals 2023 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2024. Archived from the original on 10 January 2024. Retrieved 9 January 2024.
- ^ a b "Imdelltra (AMG757)- tarlatamab-dlle kit". DailyMed. 16 May 2024. Retrieved 31 May 2024.
- ^ a b "FDA grants accelerated approval to tarlatamab-dlle for extensive stage small cell lung cancer". U.S. Food and Drug Administration (FDA). 16 May 2024. Retrieved 17 May 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "FDA approves Imdelltra (tarlatamab-dlle), the first and only T-cell engager therapy for the treatment of extensive-stage small cell lung cancer" (Press release). Amgen. 16 May 2024. Retrieved 18 May 2024 – via PR Newswire.
- ^ Research, Center for Drug Evaluation and (26 January 2022). "FDA approves tebentafusp-tebn for unresectable or metastatic uveal melanoma". FDA.
- ^ "FDA grants accelerated approval to epcoritamab-bysp for relapsed or refractory diffuse large B-cell lymphoma and high-grade B-cell lymphoma". U.S. Food and Drug Administration (FDA). 19 May 2023. Archived from the original on 23 May 2023. Retrieved 24 May 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "FDA approves treatment for relapsed or refractory diffuse large B-cell lymphoma and high-grade B-cell lymphoma". U.S. Food and Drug Administration (FDA). 19 May 2023. Archived from the original on 2 June 2023. Retrieved 2 June 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Epkinly (epcoritamab-bysp) Approved by U.S. FDA as the First and Only Bispecific Antibody to Treat Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL)" (Press release). AbbVie. 19 May 2023. Archived from the original on 19 May 2023. Retrieved 20 May 2023 – via PR Newswire.
- ^ "Drug Approval Package: Epkinly". U.S. Food and Drug Administration (FDA). 26 May 2023. Retrieved 30 May 2023.
- ^ Frampton JE (September 2023). "Epcoritamab: First Approval". Drugs. 83 (14): 1331–1340. doi:10.1007/s40265-023-01930-4. PMID 37597091. S2CID 261030074.
- ^ a b Kischel, R; et al. (2008). "Characterization in primates of MCSP- and CD33-specific human BiTE antibodies for treatment of Melanoma and AML" (PDF). Proc Am Assoc Cancer Res. 99. Abs 2404. Archived from the original (PDF) on 19 July 2011.
- ^ Lutterbuese, R; et al. (2008). "Conversion of cetuximab, panitumumab, trastuzumab and omalizumab into T-cell-engaging BiTE antibodies creates novel drug candidates of high potency" (PDF). Proc Am Assoc Cancer Res. 99. Abs 2402. Archived from the original (PDF) on 19 July 2011.
- ^ Baeuerle, PA; Reinhardt, C (2009). "Bispecific T-cell engaging antibodies for cancer therapy". Cancer Research. 69 (12): 4941–4. doi:10.1158/0008-5472.CAN-09-0547. PMID 19509221.
Further reading
[edit]- Kufer, P; Lutterbüse, R; Baeuerle, PA (2004). "A revival of bispecific antibodies". Trends in Biotechnology. 22 (5): 238–44. doi:10.1016/j.tibtech.2004.03.006. PMID 15109810.