Sex linkage
Sex linked describes the sex-specific reading patterns of inheritance and presentation when a gene mutation (allele) is present on a sex chromosome (allosome) rather than a non-sex chromosome (autosome). In humans, these are termed X-linked recessive, X-linked dominant and Y-linked. The inheritance and presentation of all three differ depending on the sex of both the parent and the child. This makes them characteristically different from autosomal dominance and recessiveness.
There are many more X-linked conditions than Y-linked conditions, since humans have several times as many genes on the X chromosome than the Y chromosome. Only females are able to be carriers for X-linked conditions; males will always be affected by any X-linked condition, since they have no second X chromosome with a healthy copy of the gene. As such, X-linked recessive conditions affect males much more commonly than females.
In X-linked recessive inheritance, a son born to a carrier mother and an unaffected father has a 50% chance of being affected, while a daughter has a 50% chance of being a carrier, however a fraction of carriers may display a milder (or even full) form of the condition due to a phenomenon known as skewed X-inactivation, in which the normal process of inactivating half of the female body's X chromosomes preferably targets a certain parent's X chromosome (the father's in this case). If the father is affected, the son will not be affected, as he does not inherit the father's X chromosome, but the daughter will always be a carrier (and may occasionally present with symptoms due to aforementioned skewed X-inactivation).
In X-linked dominant inheritance, a son or daughter born to an affected mother and an unaffected father both have a 50% chance of being affected (though a few X-linked dominant conditions are embryonic lethal for the son, making them appear to only occur in females). If the father is affected, the son will always be unaffected, but the daughter will always be affected. A Y-linked condition will only be inherited from father to son and will always affect every generation.
The inheritance patterns are different in animals that use sex-determination systems other than XY. In the ZW sex-determination system used by birds, the mammalian pattern is reversed, since the male is the homogametic sex (ZZ) and the female is heterogametic (ZW).
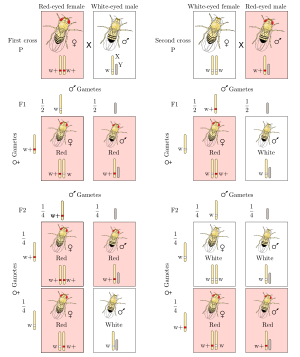
In classical genetics, a mating experiment called a reciprocal cross is performed to test if an animal's trait is sex-linked.
(A) 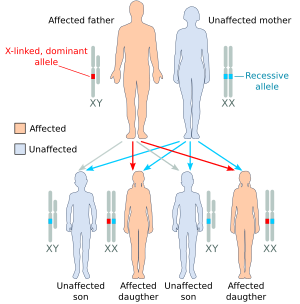
|
(B) 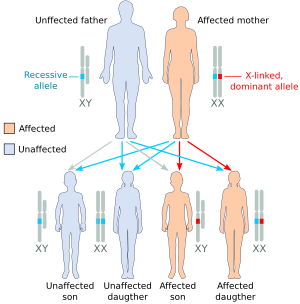
|
(C) 
|
| Illustration of some X-linked heredity outcomes (A) the affected father has one X-linked dominant allele, the mother is homozygous for the recessive allele: only daughters (all) will be affected. (B) the affected mother is heterozygous with one copy of the X-linked dominant allele: both daughters and sons will have 50% probability to be affected. (C) the heterozygous mother is called "carrier" because she has one copy of the recessive allele: sons will have 50% probability to be affected, 50% of unaffected daughters will become carriers like their mother.[2] |
X-linked dominant inheritance
[edit]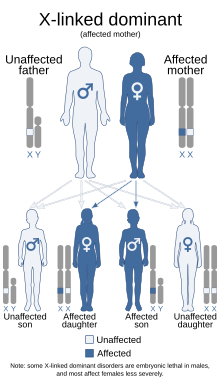
X-linked dominant inheritance occurs less frequently. Only one copy of the mutated alleles on the X chromosomes is sufficient to cause the disorder when inherited from an affected parent.
Unlike in X-linked recessive inheritance, X-linked dominant traits can affect females as much as males. Affected fathers alone will not lead to affected sons. However, if the mother is also affected, there will be a chance for the sons to be affected depending on which of the X chromosomes (recessive or dominant) is inherited. If a son displays the trait, the mother must also be affected. Some X-linked dominant traits, such as Aicardi syndrome, cause embryonic death in males, leading them to appear only in born females who continue to survive with these conditions.
X-linked recessive inheritance
[edit]
X-linked recessive inheritance is coded by the recessive version of a gene. The mutation of a gene on the X chromosome causes the phenotype to be always present in the male because they have only one X chromosome. The phenotype only occurs in a female if she is homozygous for the mutation. A female with one copy of the mutated gene is considered a carrier.
A carrier female with only one copy of the mutated gene does not often express the diseased phenotype, although X-chromosome inactivation (or skewed X-inactivation), which is common in the female population, may lead to different levels of expression.[3] There are characteristic patterns for X-linked recessive inheritance.[4] As each parent contributes one sex chromosome to their offspring, sons cannot receive the X-linked trait from affected fathers, who provide only a Y chromosome. Consequently, affected males must inherit the mutated X chromosome from their mothers. X-linked recessive traits are more common in males as they only have one X chromosome, they need only one mutated X chromosome to be affected. In contrast, females have two X chromosomes and must inherit two mutated recessive X alleles, one from each parent, to be affected. X-linked recessive phenotypes tend to skip generations.[5] A grandfather will not affect the son but could affect the grandson by passing the mutated X chromosome to his daughter who is, therefore, the carrier.
Y-linked
[edit]- Various failures in the SRY genes
Sex-linked traits in other animals
[edit]- White eyes in Drosophila melanogaster flies was one of the earliest sex-linked genes discovered.[6]
- Fur color in domestic cats: the gene that causes orange pigment is on the X chromosome; thus a Calico or tortoiseshell cat, with both black (or gray) and orange pigment, is nearly always female.
- The first sex-linked gene ever discovered was the "lacticolor" X-linked recessive gene in the moth Abraxas grossulariata by Leonard Doncaster.[7]
Related terms
[edit]It is important to distinguish between sex-linked characters, which are controlled by genes on sex chromosomes, and two other categories.[8]
Sex-influenced traits
[edit]Sex-influenced or sex-conditioned traits are phenotypes affected by whether they appear in a male or female body.[9] Even in a homozygous dominant or recessive female the condition may not be expressed fully. Example: baldness in humans.
Sex-limited traits
[edit]These are characters only expressed in one sex. They may be caused by genes on either autosomal or sex chromosomes.[9] Examples: female sterility in Drosophila; and many polymorphic characters in insects, especially in relation to mimicry. Closely linked genes on autosomes called "supergenes" are often responsible for the latter.[10][11][12]
Genetic diseases
[edit]An X-linked genetic disease is a disease inherited through a genetic defect on the X chromosome. In human cells, there is a pair of non-matching sex chromosomes, labelled X and Y. Females carry two X chromosomes, whereas males have one X and one Y chromosome. A disease or trait determined by a gene on the X chromosome demonstrates X-linked inheritance, which can be divided into dominant and recessive patterns.
The first X-linked genetic disorder described on paper was by John Dalton in 1794, then later in 1910, following Thomas Hunt Morgan's experiment, more about the sex-linked inheritance was understood. In 1961, Mary Lyon proposed the hypothesis of random X-chromosome inactivation providing the fundamental for understanding the mechanism of X-linked inheritance.
There is currently an estimation of 867 X-linked genes identified, with over 533 diseases related to X-linked genes. Common X-linked genetic diseases include Red-green colour blindness, which affects an individual's ability to see red or green images; X-linked agammaglobulinemia, resulting in a deficiency of immunity; Duchenne Muscular Dystrophy, causing muscle weakness and immobility; Hemophilia A, leading to blood clotting deficiency. X-linked recessive diseases are more frequently encountered than dominant ones and predominantly affect males, with Red-green colour blindness having the highest prevalence among all.
Genetic screening including carrier screening, prenatal screening and newborn screening could be done on individuals for early detection of genetic defects. As there are many X-linked genetic diseases, the pathology and mechanism of each varies significantly, there is no clear-cut diagnosis and treatment for all diseases. Methods of diagnosis range from blood tests to genetic tests, while treatments range from specific medications to blood infusion.
The incidence of X-linked recessive conditions in females is the square of that in males: for example, if 1 in 20 males in a human population are red–green color blind, then 1 in 400 females in the population are expected to be color-blind (1/20)*(1/20). Examples include:
- Aarskog–Scott syndrome
- Adrenoleukodystrophy (ALD)
- Bruton's agammaglobulinemia
- Color blindness
- Complete androgen insensitivity syndrome
- Congenital aqueductal stenosis (hydrocephalus)
- Duchenne muscular dystrophy
- Fabry disease
- Glucose-6-phosphate dehydrogenase deficiency
- Haemophilia A and B
- Hunter syndrome
- Inherited nephrogenic diabetes insipidus
- Menkes disease (kinky hair syndrome)
- Ornithine carbamoyltransferase deficiency
- Wiskott–Aldrich syndrome
There are fewer X-linked dominant conditions than X-linked recessive, because dominance in X-linkage requires the condition to present in females with only a fraction of the reduction in gene expression of autosomal dominance, since roughly half (or as many as 90% in some cases) of a particular parent's X chromosomes are inactivated in females.[citation needed] Examples include:
- Alport syndrome
- Coffin–Lowry syndrome (CLS)
- Fragile X syndrome
- Idiopathic hypoparathyroidism
- Incontinentia pigmenti
- Rett syndrome (RS)
- Vitamin D resistant rickets (X-linked hypophosphatemia)
Red-green colour blindness
[edit]Red-green colour blindness is a type of colour vision deficiency (CVD) caused by a mutation in X-linked genes, affecting cone cells responsible for absorbing red or green light.
The perception of red and green light is attributed to the Long (L) wavelength cones and Medium (M) wavelength cones respectively.[13] In Red-green colour blindness, mutations take place on the OPN1LW and OPN1MW genes[14] coding for the photopigments in the cones. In milder cases, those affected exhibit reduced sensitivity to red or green light, as a result of hybridisation of the genes,[14] shifting the response of one cone towards that of the other.[13] In the more extreme conditions, there is a deletion or replacement of the respective coding genes,[15] resulting in the absence of L or M cones photopigments and thus losing the ability to differentiate between red or green light completely.
X-linked agammaglobulinemia
[edit]X-linked agammaglobulinemia (XLA) is a primary immunodeficiency disorder that impairs the body’s ability to produce antibodies, which are proteins protecting us from disease-causing antigens, resulting in severe bacterial infections.[16]
XLA is associated with a mutation in the Bruton's tyrosine kinase (BTK) gene on the X chromosome,[17] which is responsible for producing BTK, an enzyme regulating B cells development.[17] B cells are a type of white blood cells essential in the production of antibodies, when at an early stage, called pre-B cells, they rely on expansion and survival signals involving BTK to mature.[18]
In affected individuals, their BTK genes have an amino acid substitution mutation,[17] altering the amino acid sequence and the structure of BTK making it faulty. Therefore, they have a normal pre-B cell counts but cannot develop mature B cells, resulting in antibody deficiency.
Duchenne Muscular Dystrophy
[edit]Duchenne Muscular Dystrophy (DMD) is a severe neuromuscular disease causing progressive weakness and damage of muscle tissues,[19] leading to mobility loss and difficulties in daily activities. In a later stage of DMD, as respiratory and cardiac muscles start to degenerate, affected individuals are likely to develop complications such as respiratory failure, cardiomyopathy and heart failure.[19]
DMD arises from a mutation, likely to be the deletion of the exons,[20][21] a nucleotide sequence in the DMD gene that codes for dystrophin. Dystrophin is a protein responsible for strengthening and stabilising muscle fibres.[22] With the loss of the dystrophin complex, the muscle cells would no longer be protected and therefore result in progressive damage or degeneration.
Haemophilia A
[edit]Haemophilia A is a blood clotting disease caused by a genetic defect in clotting factor VIII. It causes significant susceptibility to both internal and external bleeding. Individuals having more severe haemophilia can experience more frequent and intense bleeding.
Severe haemophilia A affects most patients. Patients with mild haemophilia often do not experience heavy bleeding except for surgeries and significant trauma.[23]
Screening
[edit]Carrier screening
[edit]Carrier screening aims to screen for recessive diseases. Targets of carrier screening typically do not show any symptoms but rather might have a family history of the disease or are in a stage of family planning. Carrier screening is done by performing a blood test on the individual, to identify the specific allele.[24]
Prenatal screening
[edit]Prenatal screening is offered to females during pregnancy, it involves both maternal blood tests and ultrasound to check for possible defect genes in developing fetus.[25] The screening result only confirms a possibility of genetic disease, so parents would be prepared psychologically, or could consider the option of pregnancy termination.
Newborn screening
[edit]The heel prick test is commonly used. A few drops of blood would be collected with a cotton paper from the heel of a newborn that is less than a week old,[26] samples would then be analysed for a variety of disorders.
History
[edit]Red-green colour blindness was the first X-linked genetic disorder described on paper, in 1794 by John Dalton, who is affected by the disorder himself.[27] However, it was not until later that the inheritance pattern and genetics were worked out. The X-chromosome was discovered in 1890 by Hermann Henking,[28] then in 1910, Thomas Hunt Morgan discovered an X-linked mutation on a Drosophila,[29] who then conducted experiments and observations to understand the X-linked inheritance.
In 1961, Mary Lyon proposed that one of the two X chromosomes in female mammalian cells would experience random inactivation (see X-chromosome inactivation) in the early embryonic stage.[30] According to her hypothesis, both males and females should have one single X chromosome that is active. This provided an enhancement for understanding the fundamental mechanisms of X-linked inheritance.
See also
[edit]- X-linked dominant inheritance
- X-linked recessive inheritance
- Genetic epidemiology
- List of genetic disorders
References
[edit]- ^ Morgan, Thomas Hunt 1919. The physical basis of heredity. Philadelphia: J.B. Lippincott Company.
- ^ Genetics home reference (2006), genetic conditions illustrations, National Library of Medicine.
- ^ Shvetsova, Ekaterina; Sofronova, Alina; Monajemi, Ramin; Gagalova, Kristina; Draisma, Harmen H. M.; White, Stefan J.; Santen, Gijs W. E.; Chuva de Sousa Lopes, Susana M.; Heijmans, Bastiaan T.; van Meurs, Joyce; Jansen, Rick; Franke, Lude; Kiełbasa, Szymon M.; den Dunnen, Johan T.; ‘t Hoen, Peter A. C. (14 December 2018). "Skewed X-inactivation is common in the general female population". European Journal of Human Genetics. 27 (3): 455–465. doi:10.1038/s41431-018-0291-3. ISSN 1018-4813. PMC 6460563. PMID 30552425.
- ^ Alliance, Genetic; Screening Services, The New York-Mid-Atlantic Consortium for Genetic and Newborn (8 July 2009), "INHERITANCE PATTERNS", Understanding Genetics: A New York, Mid-Atlantic Guide for Patients and Health Professionals, Genetic Alliance, retrieved 27 March 2024
- ^ Pierce, Benjamin A. (2020). Genetics: A Conceptual Approach. Macmillan Learning. pp. 154–155. ISBN 978-1-319-29714-5.
- ^ Morgan T.H. 1910. Sex-limited inheritance in Drosophila. Science 32: 120–122
- ^ Doncaster L. & Raynor G.H. 1906. Breeding experiments with Lepidoptera. Proceedings of the Zoological Society of London. 1: 125–133
- ^ Zirkle, Conway (1946). The discovery of sex-influenced, sex limited and sex-linked heredity. In Ashley Montagu M.F. (ed) Studies in the history of science and learning offered in homage to George Sarton on the occasion of his sixtieth birthday. New York: Schuman, p167–194.
- ^ a b King R.C; Stansfield W.D. & Mulligan P.K. 2006. A dictionary of genetics. 7th ed, Oxford University Press. ISBN 0-19-530761-5
- ^ Mallet J.; Joron M. (1999). "The evolution of diversity in warning color and mimicry: polymorphisms, shifting balance, and speciation". Annual Review of Ecology and Systematics. 30: 201–233. doi:10.1146/annurev.ecolsys.30.1.201.
- ^ Ford E. B. (1965) Genetic polymorphism. p17-25. MIT Press 1965.
- ^ Joron M, Papa R, Beltrán M, et al. (2006). "A conserved supergene locus controls colour pattern diversity in Heliconius butterflies". PLOS Biol. 4 (10): e303. doi:10.1371/journal.pbio.0040303. PMC 1570757. PMID 17002517.
- ^ a b Barton, Jason J. S.; Leff, Alexander; Aminoff, Michael J.; Boller, François; Swaab, D. F., eds. (2021). "Colour Vision". Neurology of vision and visual disorders. Handbook of clinical neurology. 3rd series. Vol. 178. Amsterdam, Netherlands: Elsevier. pp. 133–141. ISBN 978-0-12-821377-3. OCLC 1237102002.
- ^ a b Deeb, Samir S (1 July 2004). "Molecular genetics of colour vision deficiencies". Clinical and Experimental Optometry. 87 (4–5): 224–229. doi:10.1111/j.1444-0938.2004.tb05052.x. ISSN 0816-4622. PMID 15312026.
- ^ Neitz, J.; Neitz, M. (2011). "The genetics of normal and defective color vision". Vision Research. 51 (7): 633–651. doi:10.1016/j.visres.2010.12.002. PMC 3075382. PMID 21167193.
- ^ Smith, C. E.; Berglöf, A. (1993), Adam, M. P.; Feldman, J.; Mirzaa, G. M.; Pagon, R. A. (eds.), "X-Linked Agammaglobulinemia", GeneReviews®, Seattle (WA): University of Washington, Seattle, PMID 20301626, retrieved 27 March 2024
- ^ a b c Maas, A.; Hendriks, R. W. (2001). "Role of Bruton's tyrosine kinase in B cell development". Developmental Immunology. 8 (3–4): 171–181. doi:10.1155/2001/28962. PMC 2276078. PMID 11785667.
- ^ McDonald, C.; Xanthopoulos, C.; Kostareli, E. (2021). "The role of Bruton's tyrosine kinase in the immune system and disease". Immunology. 164 (4): 722–736. doi:10.1111/imm.13416. ISSN 0019-2805. PMC 8561098. PMID 34534359.
- ^ a b Venugopal, Vijay; Pavlakis, Steven (2024), "Duchenne Muscular Dystrophy", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29493971, retrieved 27 March 2024
- ^ Yiu, Eppie M; Kornberg, Andrew J (August 2015). "Duchenne muscular dystrophy". Journal of Paediatrics and Child Health. 51 (8): 759–764. doi:10.1111/jpc.12868. ISSN 1034-4810. PMID 25752877.
- ^ Aartsma-Rus, Annemieke; Ginjaar, Ieke B; Bushby, Kate (March 2016). "The importance of genetic diagnosis for Duchenne muscular dystrophy". Journal of Medical Genetics. 53 (3): 145–151. doi:10.1136/jmedgenet-2015-103387. ISSN 0022-2593. PMC 4789806. PMID 26754139.
- ^ Gao, Q. Q.; McNally, E. M. (17 January 2011). Terjung, Ronald (ed.). Comprehensive Physiology. Vol. 5 (1 ed.). Wiley. pp. 1223–1239. doi:10.1002/cphy.c140048. ISBN 978-0-470-65071-4. PMC 4767260. PMID 26140716.
- ^ Konkle, Barbara A.; Nakaya Fletcher, Shelley (1993). "Hemophilia A". GeneReviews®. Seattle (WA): University of Washington, Seattle. PMID 20301578.
- ^ Antonarakis, Stylianos E. (September 2019). "Carrier screening for recessive disorders". Nature Reviews Genetics. 20 (9): 549–561. doi:10.1038/s41576-019-0134-2. ISSN 1471-0056. PMID 31142809.
- ^ Cuckle, Howard; Maymon, Ron (1 February 2016). "Development of prenatal screening—A historical overview". Seminars in Perinatology. The Changing Paradigm of Perinatal screening for Birth Defects. 40 (1): 12–22. doi:10.1053/j.semperi.2015.11.003. ISSN 0146-0005. PMID 26764253.
- ^ Anderson, R.; Rothwell, E.; Botkin, J. R. (2011). "Newborn Screening". Annual Review of Nursing Research. 29 (1): 113–132. doi:10.1891/0739-6686.29.113. ISSN 0739-6686. PMC 7768912. PMID 22891501.
- ^ Hunt, David M.; Dulai, Kanwaijit S.; Bowmaker, James K.; Mollon, John D. (17 February 1995). "The Chemistry of John Dalton's Color Blindness". Science. 267 (5200): 984–988. Bibcode:1995Sci...267..984H. doi:10.1126/science.7863342. ISSN 0036-8075. PMID 7863342.
- ^ Schwartz, James (2009). In pursuit of the gene: from Darwin to DNA (1. paperback ed.). Cambridge, Mass.: Harvard Univ. Press. ISBN 978-0-674-03491-4.
- ^ Green, M M (1 January 2010). "2010: A Century of Drosophila Genetics Through the Prism of the white Gene". Genetics. 184 (1): 3–7. doi:10.1534/genetics.109.110015. ISSN 1943-2631. PMC 2815926. PMID 20061564.
- ^ DISTECHE, CHRISTINE M.; BERLETCH, JOEL B. (1 December 2015). "X-chromosome inactivation and escape". Journal of Genetics. 94 (4): 591–599. doi:10.1007/s12041-015-0574-1. ISSN 0973-7731. PMC 4826282. PMID 26690513.
