User talk:Jü
hello
[edit]in respect to the article on fluor-bi-profen, structural formula, [ http://en.wiki.x.io/w/index.php?title=File_talk:(%C2%B1)-Flurbiprofen_Structural_Formulae_V.1.svg], please be kind and edit image to display only one molecule, because most users cannot read your picture as displaying two complex molecula. Math cat`ing (talk) 12:22, 4 April 2011 (UTC)
- @Math cat`ing: fluor-bi-profen is in fact a mixture of two different molecules with different biological activity. Thus, it is correct to draw two different structures. Best regards, --Jü (talk) 18:33, 6 April 2011 (UTC)

Hello, this is a message from an automated bot. A tag has been placed on Cafea, by another Wikipedia user, requesting that it be speedily deleted from Wikipedia. The tag claims that it should be speedily deleted because Cafea is blatant advertising for a company, product, group, service or person that would require a substantial rewrite in order to become an encyclopedia article.
To contest the tagging and request that administrators wait before possibly deleting Cafea, please affix the template {{hangon}} to the page, and put a note on its talk page. If the article has already been deleted, see the advice and instructions at WP:WMD. Feel free to contact the bot operator if you have any questions about this or any problems with this bot, bearing in mind that this bot is only informing you of the nomination for speedy deletion; it does not perform any nominations or deletions itself. To see the user who deleted the page, click here CSDWarnBot (talk) 16:30, 4 February 2009 (UTC)
Hello Jü, do you know whether chlorprothixene is really cis- only? Best, Fvasconcellos (t·c) 18:04, 26 August 2009 (UTC)
- Hello Fvasconcellos, according to two sources chlorprothixene is the only the cis-isomer [(Z)-isomer]. Sources: (a) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14. Auflage (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; p. 362−363, ISBN 978-0-911910-00-1; (b) ROTE LISTE 2008, Verlag Rote Liste Service GmbH, Frankfurt am Main, p. 51, ISBN 978-3-939192-20-6.– Best --Jü (talk) 18:29, 26 August 2009 (UTC)
- OK, thanks for the information. Best, Fvasconcellos (t·c) 19:04, 26 August 2009 (UTC)
- Hello Fvasconcellos, according to two sources chlorprothixene is the only the cis-isomer [(Z)-isomer]. Sources: (a) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14. Auflage (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; p. 362−363, ISBN 978-0-911910-00-1; (b) ROTE LISTE 2008, Verlag Rote Liste Service GmbH, Frankfurt am Main, p. 51, ISBN 978-3-939192-20-6.– Best --Jü (talk) 18:29, 26 August 2009 (UTC)
Mirtazapine
[edit]Putting an image that shows both isomers is redundant and unaesthetic. A simple (RS)- or (±)- prefix in the chemical name field will do just fine. If there's something wrong with the current image, go ahead and replace it if you wish, but do not replace it with one that has two structures in it please. El3ctr0nika (talk) 20:03, 10 September 2009 (UTC)
- Hi El3ctr0nika, thanks for your kind and fast reply. Your idea is against the current IUPAC rules. For reference please read pages 1965 to 1969 of the following publication: ["Graphical representation of stereochemical configuration (IUPAC Recommendations 2006)". Pure Appl.Chem. 78 (10): 1897–1970. doi:10.1351/pac200678101897.]. Structures of BOTH enantiomers should be shown. --Jü (talk) 10:17, 11 September 2009 (UTC).
Enantiomers
[edit]This whole thing is just a stupid technical discrepancy that you need to get over. An image containing both enantiomers is not necessary and unaesthetic for the page. The vast majority of drugs are produced as racemates. Having a single isomer is rare and usually only done in special circumstances such as to get rid of certain pharmacological effects like in the case of d-amphetamine or escitalopram. Besides, on all the major chemical websites like PubChem, Chemspider, DrugBank, etc, only one enantiomer is shown. Ok? My friend Nuklear disapproves of your doings as well. El3ctr0nika (talk) 20:18, 12 September 2009 (UTC)
- Hi, I think this section is apparently correlated with the above, and I have a comment. The IUPAC recommendation Jü indicated above seems to be uncommon among chemists yet. So it would not be nice to enforce the representation manner (showing all enantiomers) in Wikipedia. In addition, putting two structures (they are very similar) on a box will confuse the readers who are not familiar with structure formula. Of course, it is important to show the compound racemic or unknown configuration, but the more important is to understand the structure itself.
- At least, figures like (±)-Nadifloxacin Enantiomers Structural Formulae.png has a little problem in accuracy, because the IUPAC recommendation states that the stereoisomers should be separated with a word like "and".
- To resolve the conflict, "and enantiomer" option will be a better choice, I think. Regards, --Calvero JP (talk) 20:45, 8 November 2009 (UTC)
- Hi Calvero JP, thanks for your comment. I do not know how to include the following text directly under the formulae of both enantiomers at Nadifloxacin: (R)-nadifloxacin (above) and (R)-nadifloxacin (below). Please try to include such a text at Nadifloxacin, this would be the best presentation. I think a modification of the file (±)-Nadifloxacin Enantiomers Structural Formulae.png would be not a good idea. In this case the file would no longer of benefit to the multilingual Wikipedia family. Best regards, --Jü (talk) 21:35, 8 November 2009 (UTC)
- Made a change at Nadifloxacin, I wish it would be appropriate. Technically, I added two entries (drug_name and image name) [1]. If the image has a single enantiomer, the correction would be completed. Thanks, --Calvero JP (talk) 12:39, 12 November 2009 (UTC)
- Thanks. Made another change at Nadifloxacin. How do you like this one? A 3rd possibility would be the following text: '(R)-Form (above) and (S)-form (below)'. However, we can also follow your suggestion and eliminate one enantiomer. Best regards, --Jü (talk) 21:54, 14 November 2009 (UTC)
- I think it looks nice. The 3rd one might be somewhat confusable, since it do not say that the chemical is a mixture. Replacing the picture would not be needed currently, although someone may do so in future. Kind regards, --Calvero JP (talk) 12:03, 15 November 2009 (UTC)
Methylphenidate stereoisomers
[edit]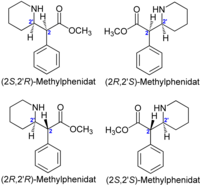
Moi, moi,
When I compare these isomers with those, they differ. The piperidines are turned over. I cannot imagine this is due to the HCL. Moreover the threo positions seem correct in the pdf (positions of phenyl and piperidine, right?).
Can you have a look at it?--Wickey-nl (talk) 15:00, 27 October 2009 (UTC)
- Moin, moin, Wickey-nl, thank you for your kind message. I have compared the absolute configurations of all compounds carefully. Both, the four drawings of Mahavir Prashad (on page 380) as well as the four stereoisomers in the file File:Methylphenidate-stereoisomers 2D-skeletal.png|thumb|200px are correct. It is just a different style of presentation. The hydrochloride does't have any influence.- Best regards, --Jü (talk) 19:55, 27 October 2009 (UTC)
- Thanks very much. I am not a chemist, so I trust your expertise. When I rotate the file alongside 180°, S,S and R,S are in a comparible position as in the pdf image. I conclude that the positioning of N on the opposite side of the 2' center makes the difference. To me the presentation of Mahavir Prashad seems less ambiguous.--Wickey-nl (talk) 08:08, 28 October 2009 (UTC)
- Moin, moin, Wickey-nl, the two formulae on top of my drawing are mirror images of each other. The two other formulae at bottom are also mirror images of each other. Such mirror images are called enantiomers. Thus, from a chemist viewpoint my entire drawing is "composed" systematically. --Jü (talk) 12:43, 28 October 2009 (UTC)
- That wasn't really what I meant. I meant the oblique 3D orientation of the ring. In any case some practise is needed.--Wickey-nl (talk) 17:19, 29 October 2009 (UTC)
Moin, moin, Jü, Still a few questions about the medicine methylphenidate.
1. Do you agree with naming this molecule as R,S-(+)-erythro-methylphenidate and certainly not Ritalin?
2. Is it possible at all, to make one of the four isomers (R,R) 100 % pure? Or does the drug always have, to a certain extent, the remainder of three isomers plus these of the used chemicals and intermediate products?--Wickey-nl (talk) 17:20, 19 November 2009 (UTC)
- Moin, moin, Wickey-nl, here are the answeres on your two questiones: (a) In my eyes the 3D-structure is not clear enough to answer your question; (b) Yes, it would be possible to "make" one of the four isomers "100% pure". Please keep in mind "100% pure" is not equivalent to "100,000000% pure". Thus, a "100 % pure isomer" in practical life may only be "99,9% pure" or "99,5% pure". However, this is the case with any chemical including gold. If you want me to cite the relevant literature for the preparation of the pure (R,R)-isomer, please ask again.--Jü (talk) 18:18, 19 November 2009 (UTC)
- (a) Did you perceive that the image on worldofmolecules.com is a Jmol applet? (I hope you have a viewer, see also the .PDB file)
(b) I was wondering how much the product of different manufacturers of the medicine can differ, how much they can economize the production on the cost of the quality and to what extent this must be controled by authorities (which is most likely not the case).--Wickey-nl (talk) 10:52, 20 November 2009 (UTC)- Moin, moin, Wickey-nl, here are the answeres on your two questiones: (a) I do not understand your comment. This might be due to my limited computer knowledge; (b) Normally it is much more economic if you do not consider the pharmacologically very important stereochemical composition. The minimum quality requirements (including stereochemical purity) for active pharmaceutical ingredients of legal commercial drugs is clearly defined in die USP (for the USA etc.) or/and in the European Pharmacopoe. Testing methods are also described. Best regards, --Jü (talk) 17:06, 24 November 2009 (UTC)
- Thanks for your answers. (a) If your browser supports .PDB files and the JMol applet, which must be the case when you see the molecule, you can turn around the molecule with the left mouse button, so you can view it well. When
 is correct the image on [2] must be wrong. (b) http://www.usp.org is a good startpoint (USA only), although Reference Standards and control are different things.--Wickey-nl (talk) 19:16, 26 November 2009 (UTC)
is correct the image on [2] must be wrong. (b) http://www.usp.org is a good startpoint (USA only), although Reference Standards and control are different things.--Wickey-nl (talk) 19:16, 26 November 2009 (UTC)
- Hallo, Wickey-nl, I have checked the configuration of both stereocenters in
 : the molecule on the left is (2S,2'S)-configurated, while the molecule on the right side is (2R,2'R)-configurated. A 1:1-mixture of the (2S,2'S)- and the (2R,2'R)-stereoisomers would be a racemate. Again, I can not detect the configuration in [3]. Best regards, --Jü (talk) 20:28, 26 November 2009 (UTC)
: the molecule on the left is (2S,2'S)-configurated, while the molecule on the right side is (2R,2'R)-configurated. A 1:1-mixture of the (2S,2'S)- and the (2R,2'R)-stereoisomers would be a racemate. Again, I can not detect the configuration in [3]. Best regards, --Jü (talk) 20:28, 26 November 2009 (UTC)
- Are you sure? According to the description the left one is R,R (dextro). In the piperidyl, N has highest CIP-priority and the opposite C the lowest (after H), right? And C on the carbonyl has highest CIP-priority. But has C in the phenyl the lowest priority or C in the piperidyl?
For JMol you must have Java installed and activated in the browser's options.--Wickey-nl (talk) 15:39, 27 November 2009 (UTC)- Hallo, you are right, the left one has (R,R)-configuration, the right one is (S,S)-configurated. Have a nice weekend --Jü (talk) 17:20, 27 November 2009 (UTC)
- I informed the webmaster of worldofmolecules and they will change it.--Wickey-nl (talk) 11:56, 4 December 2009 (UTC)
- Hallo, you are right, the left one has (R,R)-configuration, the right one is (S,S)-configurated. Have a nice weekend --Jü (talk) 17:20, 27 November 2009 (UTC)
- Are you sure? According to the description the left one is R,R (dextro). In the piperidyl, N has highest CIP-priority and the opposite C the lowest (after H), right? And C on the carbonyl has highest CIP-priority. But has C in the phenyl the lowest priority or C in the piperidyl?
- Hallo, Wickey-nl, I have checked the configuration of both stereocenters in
- Thanks for your answers. (a) If your browser supports .PDB files and the JMol applet, which must be the case when you see the molecule, you can turn around the molecule with the left mouse button, so you can view it well. When
- Moin, moin, Wickey-nl, here are the answeres on your two questiones: (a) I do not understand your comment. This might be due to my limited computer knowledge; (b) Normally it is much more economic if you do not consider the pharmacologically very important stereochemical composition. The minimum quality requirements (including stereochemical purity) for active pharmaceutical ingredients of legal commercial drugs is clearly defined in die USP (for the USA etc.) or/and in the European Pharmacopoe. Testing methods are also described. Best regards, --Jü (talk) 17:06, 24 November 2009 (UTC)
- (a) Did you perceive that the image on worldofmolecules.com is a Jmol applet? (I hope you have a viewer, see also the .PDB file)
Amlodipine
[edit]Hello Jü, an editor has asked at Talk:Amlodipine for more information on stereochemistry to be included in the article. Perhaps you can help? Best wishes, Fvasconcellos (t·c) 21:14, 10 December 2009 (UTC)
- Hallo Fvasconcellos, done! Best regards, --Jü (talk) 08:33, 22 January 2010 (UTC)
Lindlar
[edit]Although well intentioned, your image of the Lindlar catalyst is very misleading. It is not a series of compounds anymore than sodium chloride is a series of percursors. The material is quite complicated for sure, but it is probably an alloy or intermetallic with surface-bound quinoline ligands. I realize that even excellent chemists sometimes seek to present idealized views of complicated stuff, but sometimes such views are more misleading than they are helpful. Cheers,--Smokefoot (talk) 17:38, 1 January 2010 (UTC)
Rivaroxaban
[edit]Regarding your edit to rivaroxaban: In which sense has the new formula (PNG) a higher resolution than the old one (SVG)? I always thought that vector images were preferred over raster images? Just curious. --ἀνυπόδητος (talk) 14:34, 31 January 2010 (UTC)
- You are right. However, I think my formulae is an improvement regarding the presentation of the stereochemistry. --Jü (talk) 13:54, 7 February 2010 (UTC)
- Hm, I prefer images with as few atoms shown as possible, but that is only my private preference. Never mind, and thanks for your answer! ἀνυπόδητος (talk) 08:22, 8 February 2010 (UTC)
Benzene Formulas
[edit]Good idea to add this box of historical benzene formulas. But since you placed this box in the middle of the section on the ring formula of Kekule, don't you think you should add Kekule's formula to the box?Ajrocke (talk) 12:09, 20 July 2010 (UTC)
- Thanks. I shall try to add Kekule's formula to the box. Best regards, --Jü (talk) 12:53, 20 July 2010 (UTC)
- Done. --Jü (talk) 13:01, 20 July 2010 (UTC)
Vulcanization
[edit]About your nice drawing of a vulcanized polyisoprene: My impression was that there are no vinyl-S bonds, it all allylic with possibly some products of SH addition across C=C. Also your inclusion of the C2S4 ring also surprised me as the parent rings usually are not very long lived, tending to ring-open. The high S content usually is attributed to tri- and tetrasulfides beteen chains. Of course a complicating factor is that such materials are heterogeneous. But vulcanizates do obey the rules of organosulfur chemistry - vinylpolysulfides and tetrathiacyclohexanes are unhappy or at least rare. Also vulcanization is conducted at rather hot temperatures and in the presence of nucleophiles that tend to equilibrate unstable structure. So I am interested in your views on this matter. Best wishes, --Smokefoot (talk) 16:40, 17 July 2011 (UTC)
- @Smokefoot:It seems to me, that you are a specialist in this field. I have ‘painted’ the formulae after an example in the following book: Jonathan Clayden, Nick Greeves, Stuart Warren, Peter Wothers: Organic Chemistry, Orford University Press, 2001, p. 1469−1471, ISBN 978-0-19-850346-0. On page 1471 two polyisoprene chains are shown including a C2S4 cyclus and a vinyltetrasulfide substructure. In a special text book on elastomers I found a C3S2 cyclus ... Please feel free to comment. Best wishes --Jü (talk) 18:21, 17 July 2011 (UTC)
Orphaned non-free image File:DQS UL Logo.svg
[edit]
Thanks for uploading File:DQS UL Logo.svg. The image description page currently specifies that the image is non-free and may only be used on Wikipedia under a claim of fair use. However, the image is currently not used in any articles on Wikipedia. If the image was previously in an article, please go to the article and see why it was removed. You may add it back if you think that that will be useful. However, please note that images for which a replacement could be created are not acceptable for use on Wikipedia (see our policy for non-free media).
Note that any non-free images not used in any articles will be deleted after seven days, as described in the criteria for speedy deletion. Thank you. Skier Dude (talk) 05:34, 8 October 2011 (UTC)
Which one do you think is better?
[edit]

--Smokefoot (talk) 22:47, 18 February 2012 (UTC)
- Hallo Smokefoot, thanks for your question. I took a new look on both images and decided to adopt some of the content of the image below in a new image which will be uploaded in about 15 minutes and will also be shown on this page. After this you may decide to replace it by your own picture if you feel this is more instructive than mine. Have a nice start in the new week! Best regards, --Jü (talk) 10:24, 19 February 2012 (UTC)


The article Envelope conformation has been proposed for deletion because of the following concern:
- no indication of being notable in its own right - might be better in the article on Cyclopentane.
While all contributions to Wikipedia are appreciated, content or articles may be deleted for any of several reasons.
You may prevent the proposed deletion by removing the {{proposed deletion/dated}} notice, but please explain why in your edit summary or on the article's talk page.
Please consider improving the article to address the issues raised. Removing {{proposed deletion/dated}} will stop the proposed deletion process, but other deletion processes exist. In particular, the speedy deletion process can result in deletion without discussion, and articles for deletion allows discussion to reach consensus for deletion. noq (talk) 18:18, 23 September 2012 (UTC)
Chemical structure images
[edit]Hi Jü. First, I would like to say that I like your chemical structure images. It is good to have your improvements from lower quality .gif and .png images to your higher quality .svg images. However, I have noticed in many cases that you replace existing high quality .svg images with yours. I presume that you are doing this because you prefer the style where skeletal formulas terminate with CH3 rather than just the end of a line. This is a matter of style that seems to differ on the English Wikipedia compared to the German Wikipedia, your home wiki. Based on comparison to policies such as WP:ENGVAR or WP:BCE, which essentially forbid changing from one style to another person's preferred style without consensus, when the quality of the image is not an improvement, it is a bad idea to change images solely on matters of style. So I would like to ask you to stop doing this unless there is consensus to do so at WT:CHEM or WT:CHEM. (And, likewise, if I went to the German Wikipedia and started replacing images like File:Pentylamine Structural Formula V.1.svg with images like File:Pentylamine.svg, I would expect to be asked to stop.) Thank you. ChemNerd (talk) 15:53, 26 July 2013 (UTC)
- See also on WP:CSDG#General, bullets 5 and 6. --Leyo 22:18, 26 July 2013 (UTC)
- Hi ChemNerd, thanks for your positive response and your additional remarks regarding skeletal formulas terminate with CH3 rather than just the end of a line. If one organic chemist (like me) is communicating with a colleague of the same profession, the sketal formulae without an explicit CH3 is often preferred, thats true. However, I want a broader audience to understand me, especially if I write in an encyclopedia. This is not only my personal taste, please comment on WP:CSDG#General, bullets 5 and 6.– Have a nice weekend. Thank you. --Jü (talk) 12:19, 27 July 2013 (UTC)
- I'm sorry if I wasn't clear. I realize that neither style is preferred in policy - WP:CSDG makes it clear that both are correct and acceptable - and that is why edits such as this aren't a good idea in my opinion. That is why I reverted the edit, but I think my edit summary wasn't correct as written. Individual chemists here seem to prefer the fully skeletal style. Simply switching from an .svg in one style to the other style doesn't make an improvement and just risks irritating the majority here. That is the reason that it is not acceptable on the English Wikipedia to make spelling changes between two acceptable spellings (such as "color" to "colour", for example) without consensus - it may irritate some people without making any real improvement. The spirit of that rule should be followed in this situation, in my opinion. Regards, ChemNerd (talk) 11:57, 28 July 2013 (UTC)
- Hi ChemNerd, thanks for your positive response and your additional remarks regarding skeletal formulas terminate with CH3 rather than just the end of a line. If one organic chemist (like me) is communicating with a colleague of the same profession, the sketal formulae without an explicit CH3 is often preferred, thats true. However, I want a broader audience to understand me, especially if I write in an encyclopedia. This is not only my personal taste, please comment on WP:CSDG#General, bullets 5 and 6.– Have a nice weekend. Thank you. --Jü (talk) 12:19, 27 July 2013 (UTC)
Competition between aldol and Claisen condensation
[edit]Hi Jü, we have met a couple of times before on the Dutch version of Wikipedia and I know you are quite acquainted with organic chemistry. I contact you in order to get something clarified. I am currently reading the book 'Classics in Total Synthesis' (1996) by Nicolaou and Sorensen in order to gain some background information that could be used on Wikipedia (and also to train myself in comprehending total synthesis strategies). I came across the total synthesis of monensin by Kishi, published in JACS in 1979: it is a very convergent one, that uses a particular interesting late-stage aldol reaction, namely:

The left wing of the molecule possesses two potential electrophilic sites: an aldehyde (indicated in blue) and an ester (indicated in green). What fascinates me (and also the authors of the book) is the fact that the aldehyde chemoselectively reacts with the methyl ketone enolate. The ester functionality remains untouched. Then, I was thinking a bit further, and wondered whether this also could happen when the aldehyde was a ketone, and thus: to what extend the competition between aldol reaction and Claisen reaction applies. Furthermore, if this would be an intramolecular process, the Baldwin rules have to be applied. To give a more simple example:

I would expect that there will be some competition on this substrate: both the ester and the ketone are electrophilic. Another contributing factor here could be the ring size (according to Baldwin rules, enolendo-exo-trig cyclization is disfavored for 3-5 membered rings, which would imply that the Claisen condensation is more important and the seven membered ring is formed; but on the other hand: entropy discourages the formation of larger rings). I have searched some literature via SciFinder, because I would like to expand the articles on Wikipedia concerning aldol and Claisen competition, and found one paper where it was the other way around: the aldol reaction (and subsequent condensation to the five-membered enone) occurs, while the ester remains untouched (in the example above, the ester is even more hindered by the adjacent quaternary center and perhaps this could further drive the reaction towards the aldol reaction). Could you somehow explain the outcome of such events and help me to understand this phenomenon? Best regards, Capaccio (talk) 16:08, 4 January 2014 (UTC)
- Hi Capaccio, it's nice to "meet" you again... It seems to me, you have a deeper understanding of the discussed phenomena on the Aldol Claisen competition compared to me. Thus, an advice from my side may not lead to an improved understanding. Sorry. Have a wonderful New Year! --Jü (talk) 18:06, 6 January 2014 (UTC)
- Hi Jü, thanks for your honest answer! In the mean time I think I have found some clues to this issue, namely the electronic effects of the ketone carbonyl group vs the ester carbonyl group. The aldol reaction will be faster due to this effect. Furthermore, kinetic control of the enolate formation will further accentuate this and drive the reaction towards the enone. In a thermodynamically controlled process, equilibration is allowed to occur, which will lead to mixtures of enones and diketones. But this also depends on the substrate (steric hindrance, ring size, side reactions, ...). I whish you all the best for 2014! Best regards, Capaccio (talk) 19:19, 6 January 2014 (UTC)
- The Classics discussion ("15.2 Retrosynthetic Analysis and Strategy", p230-232) of that left-hand structure notes that "the aldehydic function at C-7 is inherently more reactive, and thus more susceptible to a nucleophilic attack, than the methoxycarbonyl group at C-1." That's a well-known pattern of carbonyl functional-group reactivity difference for all types of nucleophiles. It doesn't seem to be mentioned in Carbonyl#Reactivity, which is a serious omission. DMacks (talk) 22:06, 6 January 2014 (UTC)
- Exactly, but then how do ketones fit in this picture (like in the example a gave above)? Ketones are less prone to nucleophilic attack than aldehydes due to the extra inductive effect of the other alkyl/aryl side chain, right? - Capaccio (talk) 11:52, 7 January 2014 (UTC)
- Ketones are indeed less prone to attack than aldehydes for various steric and electronic reasons. But ketones are still more prone to attack than esters in general, though I assume one could design a system where the ketone was so hindered that attack was very difficult and/or where small electron-withdrawing groups could increase the reactivity of the ester...maybe even enough to reverse the selectivity. Your second example diagram also has the rings fused, not just Baldwin's rules ring-closure concerns. DMacks (talk) 12:33, 7 January 2014 (UTC)
- Thanks for your help! As I may conclude from your explanation, the reaction above will be in favour of the aldol reaction and not the Claisen reaction, due to the electronic effect of the ketone vs. ester carbonyl (the ester 'alkoxy part' exerts a slight mesomeric effect onto the carbonyl, while in ketones only inductive effects play a role). But, if the ketone would be more hindered (e.g. dimethylated on the alfa position), the nucleophilic attack will perhaps become more difficult and the focus would shift towards Claisen. I read the paper about Baldwin rules (Tetrahedron, Volume 38, Issue 19, 1982, Pages 2939–2947) and saw that only acyclic substrates were used overthere, while the one I suggested above is already cyclic (and thus implies a certain pre-arrangement of the functional groups). Best regards, Capaccio (talk) 13:01, 7 January 2014 (UTC)
- Ketones are indeed less prone to attack than aldehydes for various steric and electronic reasons. But ketones are still more prone to attack than esters in general, though I assume one could design a system where the ketone was so hindered that attack was very difficult and/or where small electron-withdrawing groups could increase the reactivity of the ester...maybe even enough to reverse the selectivity. Your second example diagram also has the rings fused, not just Baldwin's rules ring-closure concerns. DMacks (talk) 12:33, 7 January 2014 (UTC)
- Exactly, but then how do ketones fit in this picture (like in the example a gave above)? Ketones are less prone to nucleophilic attack than aldehydes due to the extra inductive effect of the other alkyl/aryl side chain, right? - Capaccio (talk) 11:52, 7 January 2014 (UTC)
- The Classics discussion ("15.2 Retrosynthetic Analysis and Strategy", p230-232) of that left-hand structure notes that "the aldehydic function at C-7 is inherently more reactive, and thus more susceptible to a nucleophilic attack, than the methoxycarbonyl group at C-1." That's a well-known pattern of carbonyl functional-group reactivity difference for all types of nucleophiles. It doesn't seem to be mentioned in Carbonyl#Reactivity, which is a serious omission. DMacks (talk) 22:06, 6 January 2014 (UTC)
- Hi Jü, thanks for your honest answer! In the mean time I think I have found some clues to this issue, namely the electronic effects of the ketone carbonyl group vs the ester carbonyl group. The aldol reaction will be faster due to this effect. Furthermore, kinetic control of the enolate formation will further accentuate this and drive the reaction towards the enone. In a thermodynamically controlled process, equilibration is allowed to occur, which will lead to mixtures of enones and diketones. But this also depends on the substrate (steric hindrance, ring size, side reactions, ...). I whish you all the best for 2014! Best regards, Capaccio (talk) 19:19, 6 January 2014 (UTC)
Software used to create File:Phenylethyl_Amine_General_Formula_V1.svg
[edit]I love this structure of yours, [1], but I would like to know which software you used, the reason being is that I would like to create a structure that's similar for my own Wiki (from Wikia). Vielen Dank für Ihre Zeit (I hope that means in German, "Thank you for time"). Brenton (contribs · email · talk · uploads) 06:22, 12 September 2014 (UTC)
- Thanks, the structure was drawn using ChemDraw Pro 12.0 with a Mac OS 10.9. Your German is perfect. Do you need assistance? Please do net hesitate to ask me once again. Viele Grüße --Jü (talk) 09:45, 13 September 2014 (UTC)
Please see
[edit]The following Talk entry, [4], thank you. Le Prof Leprof 7272 (talk) 21:43, 16 July 2015 (UTC)
Orphaned non-free image File:Logo DQS-UL.jpg
[edit]
Thanks for uploading File:Logo DQS-UL.jpg. The image description page currently specifies that the image is non-free and may only be used on Wikipedia under a claim of fair use. However, the image is currently not used in any articles on Wikipedia. If the image was previously in an article, please go to the article and see why it was removed. You may add it back if you think that that will be useful. However, please note that images for which a replacement could be created are not acceptable for use on Wikipedia (see our policy for non-free media).
Note that any non-free images not used in any articles will be deleted after seven days, as described in the criteria for speedy deletion. Thank you. --B-bot (talk) 17:49, 20 September 2015 (UTC)
Nomination of Zoltan Hajos for deletion
[edit]A discussion is taking place as to whether the article Zoltan Hajos is suitable for inclusion in Wikipedia according to Wikipedia's policies and guidelines or whether it should be deleted.
The article will be discussed at Wikipedia:Articles for deletion/Zoltan Hajos until a consensus is reached, and anyone is welcome to contribute to the discussion. The nomination will explain the policies and guidelines which are of concern. The discussion focuses on high-quality evidence and our policies and guidelines.
Users may edit the article during the discussion, including to improve the article to address concerns raised in the discussion. However, do not remove the article-for-deletion notice from the top of the article.
I removed the CSD G10 tag because it didn't appear to be an attack page, as Wikipedia understands it. But as an account identifying itself as the subject of the article has asked for its deletion, it warrants further discussion. I hope you can contribute to the deletion discussion. Liz Read! Talk! 16:07, 6 October 2015 (UTC)
Disambiguation link notification for October 27
[edit]Hi. Thank you for your recent edits. Wikipedia appreciates your help. We noticed though that when you edited Wolffenstein–Böters reaction, you added a link pointing to the disambiguation page Richard Wolffenstein. Such links are almost always unintended, since a disambiguation page is merely a list of "Did you mean..." article titles. Read the FAQ • Join us at the DPL WikiProject.
It's OK to remove this message. Also, to stop receiving these messages, follow these opt-out instructions. Thanks, DPL bot (talk) 11:31, 27 October 2015 (UTC)
![]() Done
Done
Hi,
You appear to be eligible to vote in the current Arbitration Committee election. The Arbitration Committee is the panel of editors responsible for conducting the Wikipedia arbitration process. It has the authority to enact binding solutions for disputes between editors, primarily related to serious behavioural issues that the community has been unable to resolve. This includes the ability to impose site bans, topic bans, editing restrictions, and other measures needed to maintain our editing environment. The arbitration policy describes the Committee's roles and responsibilities in greater detail. If you wish to participate, you are welcome to review the candidates' statements and submit your choices on the voting page. For the Election committee, MediaWiki message delivery (talk) 13:59, 24 November 2015 (UTC)
![]() Done
Done
Disambiguation link notification for January 23
[edit]Hi. Thank you for your recent edits. Wikipedia appreciates your help. We noticed though that when you edited L-DOPA, you added a link pointing to the disambiguation page Tlc. Such links are almost always unintended, since a disambiguation page is merely a list of "Did you mean..." article titles. Read the FAQ • Join us at the DPL WikiProject.
It's OK to remove this message. Also, to stop receiving these messages, follow these opt-out instructions. Thanks, DPL bot (talk) 10:07, 23 January 2016 (UTC)
![]() Done
Done
Thank you for being one of Wikipedia's top medical contributors!
[edit]- please help translate this message into the local language

|
The Cure Award |
| In 2015 you were one of the top 300 medical editors across any language of Wikipedia. Thank you from Wiki Project Med Foundation for helping bring free, complete, accurate, up-to-date health information to the public. We really appreciate you and the vital work you do! Wiki Project Med Foundation is a user group whose mission is to improve our health content. Consider joining here, there are no associated costs, and we would love to collaborate further. |
Thanks again :) -- Doc James along with the rest of the team at Wiki Project Med Foundation 03:59, 29 February 2016 (UTC)
ArbCom Elections 2016: Voting now open!
[edit]Hello, Jü. Voting in the 2016 Arbitration Committee elections is open from Monday, 00:00, 21 November through Sunday, 23:59, 4 December to all unblocked users who have registered an account before Wednesday, 00:00, 28 October 2016 and have made at least 150 mainspace edits before Sunday, 00:00, 1 November 2016.
The Arbitration Committee is the panel of editors responsible for conducting the Wikipedia arbitration process. It has the authority to impose binding solutions to disputes between editors, primarily for serious conduct disputes the community has been unable to resolve. This includes the authority to impose site bans, topic bans, editing restrictions, and other measures needed to maintain our editing environment. The arbitration policy describes the Committee's roles and responsibilities in greater detail.
If you wish to participate in the 2016 election, please review the candidates' statements and submit your choices on the voting page. MediaWiki message delivery (talk) 22:08, 21 November 2016 (UTC)
{{Erledigt|Jü (talk) 18:35, 1 April 2017 (UTC)}}
Emanuel Gil-Av moved to draftspace
[edit]An article you recently created, Emanuel Gil-Av, does not have enough sources and citations as written to remain published. It needs more citations from reliable, independent sources. (?) Information that can't be referenced should be removed (verifiability is of central importance on Wikipedia). I've moved your draft to draftspace (with a prefix of "Draft:" before the article title) where you can incubate the article with minimal disruption. When you feel the article meets Wikipedia's general notability guideline and thus is ready for mainspace, please follow the prompts on the Articles for Creation template atop the page. Justlettersandnumbers (talk) 22:52, 18 July 2017 (UTC)
Your submission at Articles for creation: Emanuel Gil-Av (July 24)
[edit]
- If you would like to continue working on the submission, go to Draft:Emanuel Gil-Av and click on the "Edit" tab at the top of the window.
- If you need any assistance, you can ask for help at the Articles for creation help desk or on the reviewer's talk page.
- You can also use Wikipedia's real-time chat help from experienced editors.

|
Hello! Jü,
I noticed your article was declined at Articles for Creation, and that can be disappointing. If you are wondering why your article submission was declined, please post a question at the Articles for creation help desk. If you have any other questions about your editing experience, we'd love to help you at the Teahouse, a friendly space on Wikipedia where experienced editors lend a hand to help new editors like yourself! See you there! 97198 (talk) 10:36, 24 July 2017 (UTC)
|
Your submission at Articles for creation: Emanuel Gil-Av has been accepted
[edit]
The article has been assessed as Start-Class, which is recorded on the article's talk page. You may like to take a look at the grading scheme to see how you can improve the article.
You are more than welcome to continue making quality contributions to Wikipedia. Note that because you are a logged-in user, you can create articles yourself, and don't have to post a request. However, you may continue submitting work to Articles for Creation if you prefer.
- If you have any questions, you are welcome to ask at the help desk.
- If you would like to help us improve this process, please consider .
Thank you for helping improve Wikipedia!
SwisterTwister talk 18:18, 28 July 2017 (UTC)
The article Deutsche Extrakt Kaffee has been proposed for deletion because of the following concern:
Fails WP:NCORP. All I can find are Bloomberg-esque entries which confer no notability.
While all constructive contributions to Wikipedia are appreciated, pages may be deleted for any of several reasons.
You may prevent the proposed deletion by removing the {{proposed deletion/dated}} notice, but please explain why in your edit summary or on the article's talk page.
Please consider improving the page to address the issues raised. Removing {{proposed deletion/dated}} will stop the proposed deletion process, but other deletion processes exist. In particular, the speedy deletion process can result in deletion without discussion, and articles for deletion allows discussion to reach consensus for deletion. DrStrauss talk 20:31, 11 October 2017 (UTC)
ArbCom 2017 election voter message
[edit]Hello, Jü. Voting in the 2017 Arbitration Committee elections is now open until 23.59 on Sunday, 10 December. All users who registered an account before Saturday, 28 October 2017, made at least 150 mainspace edits before Wednesday, 1 November 2017 and are not currently blocked are eligible to vote. Users with alternate accounts may only vote once.
The Arbitration Committee is the panel of editors responsible for conducting the Wikipedia arbitration process. It has the authority to impose binding solutions to disputes between editors, primarily for serious conduct disputes the community has been unable to resolve. This includes the authority to impose site bans, topic bans, editing restrictions, and other measures needed to maintain our editing environment. The arbitration policy describes the Committee's roles and responsibilities in greater detail.
If you wish to participate in the 2017 election, please review the candidates and submit your choices on the voting page. MediaWiki message delivery (talk) 18:42, 3 December 2017 (UTC)
1-Octen-3-ol
[edit]Hello Jü, first of all, I really appreciate your work here at Wikipedia, I like your uploaded images especially. The reason I'm writing this post is that I stumbled upon one of those images, namely on the page of oct-1-en-3-ol [[5]] and I compared it to other sources. Are you sure that's the correct structure of oct-1-en-3-ol? I just checked nist.gov, and their page shows an image without the dashed wedge hydrogen atom (link). Then I checked CRC Handbook of Chemistry and Physics (98th Edition) and found the same image there, without the wedged H. After that I drew the structure of oct-1-en-3-ol without the wedged H in a PC program called "ACD/Chemsketch" and used the "Generate name" function. It showed the following result: "oct-1-en-3-ol". Then I went and drew the structure, but this time with the wedged hydrogen atom, just like in your image. Again called the Generate Name function, and the result was: "(3R)-oct-1-en-3-ol". So, is it possible that the current image at oct-1-en-3-ol page [[6]] shows one of the two enantiomers instead of generic oct-1-en-3-ol? If so, wouldn't it be better to show both enantiomers instead of just one?
Excuse me if I misunderstood something here, I'm new at chemistry, so I might be wrong. Assaiki (talk) 19:17, 19 January 2018 (UTC)
- Hello Assaiki, your interpretation is correct. I have replaced the structure of (R)-oct-1-en-3-ol on the page of oct-1-en-3-ol [[7]] by both structures (R)-oct-1-en-3-ol and (S)-oct-1-en-3-ol. I hope this is okay for you. In de:Wikipedia we use the same formulae, see: [[8]] Best regards, --Jü (talk) 19:00, 21 January 2018 (UTC)
ArbCom 2018 election voter message
[edit]Hello, Jü. Voting in the 2018 Arbitration Committee elections is now open until 23.59 on Sunday, 3 December. All users who registered an account before Sunday, 28 October 2018, made at least 150 mainspace edits before Thursday, 1 November 2018 and are not currently blocked are eligible to vote. Users with alternate accounts may only vote once.
The Arbitration Committee is the panel of editors responsible for conducting the Wikipedia arbitration process. It has the authority to impose binding solutions to disputes between editors, primarily for serious conduct disputes the community has been unable to resolve. This includes the authority to impose site bans, topic bans, editing restrictions, and other measures needed to maintain our editing environment. The arbitration policy describes the Committee's roles and responsibilities in greater detail.
If you wish to participate in the 2018 election, please review the candidates and submit your choices on the voting page. MediaWiki message delivery (talk) 18:42, 19 November 2018 (UTC)
ArbCom 2019 election voter message
[edit]ArbCom 2020 Elections voter message
[edit]ArbCom 2021 Elections voter message
[edit]Orphaned non-free image File:DQS-global-LOGO.png
[edit]
Thanks for uploading File:DQS-global-LOGO.png. The image description page currently specifies that the image is non-free and may only be used on Wikipedia under a claim of fair use. However, the image is currently not used in any articles on Wikipedia. If the image was previously in an article, please go to the article and see why it was removed. You may add it back if you think that that will be useful. However, please note that images for which a replacement could be created are not acceptable for use on Wikipedia (see our policy for non-free media).
Note that any non-free images not used in any articles will be deleted after seven days, as described in section F5 of the criteria for speedy deletion. Thank you. --B-bot (talk) 16:05, 8 June 2022 (UTC)
I have sent you a note about a page you started
[edit]Hello, Jü. Thank you for your work on Ravi Bhushan. User:SunDawn, while examining this page as a part of our page curation process, had the following comments:
Thanks for creating the article!
To reply, leave a comment here and begin it with {{Re|SunDawn}}. Please remember to sign your reply with ~~~~. (Message delivered via the Page Curation tool, on behalf of the reviewer.)
✠ SunDawn ✠ (contact) 12:19, 23 October 2022 (UTC)
- Hi SunDawn, thanks for your kind words. Best regards, --Jü (talk) 16:34, 24 October 2022 (UTC)
Chiral TLC
[edit]Thanks for the addition of doi:10.1016/j.chroma.2009.12.071 to thin-layer chromatography. Definitely a useful low-intensity alternative to chiral HPLC for qualitative work. Do you think this should be more centrally placed, such as in chiral column chromatography (a tiny stub at the moment), with that article renamed to chiral chromatography? Already that article includes chiral GC, whereas column chromatography appears to restrict the term column chromatography to liquid mobile phases. DMacks (talk) 15:31, 24 October 2022 (UTC)
- Hi DMacks, thanks. I think a special article named chiral thin-layer chromatography would be a good idea. I have several references for such papers covering chral TLC. Best regards, --Jü (talk) 16:31, 24 October 2022 (UTC)
- Hi DMacks, a collegue has written his first Wikipedia article entitled chiral thin-layer chromatography. He has uploaded the short article into the review process for beginners in the en:Wikipedia yesterday or one day before. Maybe, you can accelerate the review process? Best regards, --Jü (talk) 08:40, 28 October 2022 (UTC)
- Hi DMacks, thanks! Best regards, --Jü (talk) 20:05, 29 November 2022 (UTC)
- Hi DMacks, a collegue has written his first Wikipedia article entitled chiral thin-layer chromatography. He has uploaded the short article into the review process for beginners in the en:Wikipedia yesterday or one day before. Maybe, you can accelerate the review process? Best regards, --Jü (talk) 08:40, 28 October 2022 (UTC)
ArbCom 2022 Elections voter message
[edit]Hello! Voting in the 2022 Arbitration Committee elections is now open until 23:59 (UTC) on Monday, 12 December 2022. All eligible users are allowed to vote. Users with alternate accounts may only vote once.
The Arbitration Committee is the panel of editors responsible for conducting the Wikipedia arbitration process. It has the authority to impose binding solutions to disputes between editors, primarily for serious conduct disputes the community has been unable to resolve. This includes the authority to impose site bans, topic bans, editing restrictions, and other measures needed to maintain our editing environment. The arbitration policy describes the Committee's roles and responsibilities in greater detail.
If you wish to participate in the 2022 election, please review the candidates and submit your choices on the voting page. If you no longer wish to receive these messages, you may add {{NoACEMM}} to your user talk page. MediaWiki message delivery (talk) 00:39, 29 November 2022 (UTC)
ArbCom 2023 Elections voter message
[edit]Hello! Voting in the 2023 Arbitration Committee elections is now open until 23:59 (UTC) on Monday, 11 December 2023. All eligible users are allowed to vote. Users with alternate accounts may only vote once.
The Arbitration Committee is the panel of editors responsible for conducting the Wikipedia arbitration process. It has the authority to impose binding solutions to disputes between editors, primarily for serious conduct disputes the community has been unable to resolve. This includes the authority to impose site bans, topic bans, editing restrictions, and other measures needed to maintain our editing environment. The arbitration policy describes the Committee's roles and responsibilities in greater detail.
If you wish to participate in the 2023 election, please review the candidates and submit your choices on the voting page. If you no longer wish to receive these messages, you may add {{NoACEMM}} to your user talk page. MediaWiki message delivery (talk) 00:35, 28 November 2023 (UTC)

