Urokinase
| File:1VJA.png | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| DrugBank | |
| Chemical and physical data | |
| Formula | C1376H2145N383O406S18 |
| Molar mass | 31126.5 g/mol g·mol−1 |
| plasminogen activator, urokinase | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | PLAU | ||||||
| NCBI gene | 5328 | ||||||
| HGNC | 9052 | ||||||
| OMIM | 191840 | ||||||
| RefSeq | NM_002658 | ||||||
| UniProt | P00749 | ||||||
| Other data | |||||||
| EC number | 3.4.21.31 | ||||||
| Locus | Chr. 10 q24 | ||||||
| |||||||
Urokinase (Abbokinase), also called urokinase-type Plasminogen Activator (uPA), is a serine protease (EC 3.4.21.73). Urokinase was originally isolated from human urine, but is present in several physiological locations, such as blood stream and the extracellular matrix. The primary physiological substrate is plasminogen, which is an inactive zymogen form of the serine protease plasmin. Activation of plasmin triggers a proteolysis cascade which, depending on the physiological environment participate in thrombolysis or extracellular matrix degradation. This links urokinase to vascular diseases and cancer.
Molecular characteristics
Urokinase is a 411 residue protein, consisting of three domains: the serine protease domain, the kringle domain and the growth factor domain. Urokinase is synthesized as a zymogen form (prourokinase or single chain urokinase), and is activated by proteolytic cleavage between L158 and I159. The two resulting chains are kept together by a disulfide bond.
Interaction partners
The most important inhibitors of urokinase are the serpins plasminogen activator inhibitor-1 (PAI-1) and plasminogen activator inhibitor-2 (PAI-2), which inhibits the protease activity irreversibly. In the extracellular matrix urokinase is tethered to the cell membrane by its interaction to the urokinase receptor.
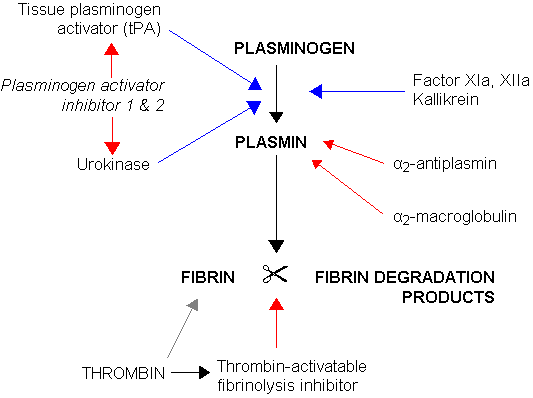
Urokinase and cancer
Elevated expression levels of urokinase and several other components of the plasminogen activation system are found to be correlated with tumor malignancy. It is believed that the tissue degradation following plasminogen activation, facilitates tissue invasion and thus contributes to metastasis. This makes urokinase an attractive drug target and inhibitors have been sought to be used as anticancer agents. However incompatibilities between the human and murine system hampers clinical evaluation of these agents. Through its interaction with the urokinase receptor, urokinase affects several other aspects of cancer biology such as cells adhesion, migration and cellular mitotic pathways.
Clinical applications
Urokinase is used clinically as a thrombolytic agent in the treatment of severe or massive deep venous thrombosis, pulmonary embolism, myocardial infarction, and occluded intravenous or dialysis cannulas. It is also administered intrapleurally to improve the drainage of complicated pleural effusions and empyemas. Urokinase is presently marketed as KinlyticTM, and competes with AlteplaseTM as a thrombolytic drug in infarctation.
