Triflic acid
| Trifluoromethanesulfonic acid | |||
|---|---|---|---|
| |||
| General | |||
| Systematic name | Trifluoromethanesulfonic acid | ||
| Other names | Triflic Acid | ||
| Molecular formula | CF3SO3H | ||
| Acidic power | - 14.9 | ||
| Molar mass | 150.08 g/mole | ||
| Appearance | limpid liquid | ||
| CAS number | |||
| Properties | |||
| Density and phase | 1.696 g cm–3 | ||
| Solubility in water | Completely miscible | ||
| Freezing Point | -40.0°C at 760 mmHg | ||
| Boiling point | 162 °C at 760 mmHg | ||
| Hazards | |||
| MSDS | |||
| Main hazards | Corrosive, eye irritant | ||
| Flash point | Non-flammable | ||
| R/S statement | R: none S: none | ||
| RTECS number | |||
| Related compounds | |||
| Related acids | FSO3H H2SO4 | ||
| Other cations | |||
| Related salts | CF3SO3Li CF3SO3Na CF3SO3K | ||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |||
Trifluoromethanesulfonic acid, also known as triflic acid or TfOH, is a sulfonic acid with the chemical formula CF3SO3H . It is often regarded as one of the strongest acids, and is one of a number of so-called "superacids". It is about 1000 times stronger than sulfuric acid. Triflic acid is widely used especially as a catalyst and a precursor in organic chemistry [1] [2].
Properties
Triflic acid is a hygroscopic, colorless liquid at room temperature. It is soluble in polar solvents such as DMF, DMSO, acetonitrile, and dimethyl sulfone. Addition of triflic acid to polar solvents can be dangerously exothermic.
With an Ka = 8.0 *1014 mole kg-1, HOTf qualifies as a superacid. Triflic acid owes many of its useful properties to its great thermal and chemical stability. Both the acid and its conjugate base CF3SO3-, known as triflate, resist oxidation/reduction reactions, whereas many strong acids are oxidizing, e.g. HClO4 and HNO3. The triflate anion is immune to attack by even strong nucleophiles. Because of it resistance to oxidation and reduction, triflic acid is a very useful and versatile reagent. Further recommending its use, triflic acid does not sulfonate substrates, which can be a problem with sulfuric acid, fluorosulfuric acid, and chlorosulfonic acid. Below is a prototypical sulfonation, which HOTf does not undergo:
- C6H6 + H2SO4 → C6H5(SO3H) + H2O
Triflic acid fumes in moist air and forms a stable solid monohydrate, CF3SO3H*H2O, melting point 34 °C.
History and syntheses
Trifluoromethanesulfonic acid was first synthesized in 1954 by Haszeldine and Kidd by the following reaction:(1)
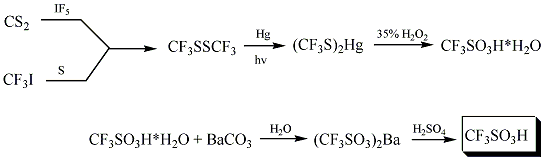
Other ways to synthesize trifluoromethanesulfonic acid including electrochemical fluorination (aka ECF)(1)

The industrial synthesis involves hydrolysis of CF3SO2F, followed by acidification. Triflic acid is purified by distillation with a small amount of Tf2O.
Uses
Triflic acid is useful in protonations because the conjugate base of triflic acid will not react with other reagents.
Salt formation
Trifluoromethanesulfonic acid exothermically reacts with metal carbonates and hydroxides. Illustrative is the synthesis of Cu(OTf)2(1).
- CuCO3 + 2 CF3SO3H → Cu(O3SCF3)2 + H2O + CO2
Far more interesting to the synthetic chemist is the conversion of chloro complexes to the corresponding triflates. Illustrative is the synthesis of [Co(NH3)5OTf]2+:
- 3 CF3SO3H + [Co(NH3)5Cl]Cl2 → [Co(NH3)5O3SCF3](O3SCF3)2 + 3 HCl
This conversion is conducted in neat HOTf at 100 °C, followed by precipitation of the salt by the addition of ether.
Organic reactions
Triflic acid reacts with acyl halides to give mixed anhydrides, which are strong acylating agents, e.g. in Friedel-Crafts reactions.(1)
- CH3C(O)Cl + CF3SO3H → CH3C(O)OSO2CF3 + HCl
- CH3C(O)OSO2CF3 + C6H6 → CH3C(O)C6H5 + CF3SO3H
Triflic acid catalyzes the reaction of aromatic compounds with sulfonyl chlorides, probably also via the intermediacy of a mixed anhydride.(1)
This is a very similar reaction to what would be done if one wanted to create polymers using triflic acid in the synthesis. Other Friedel-Crafts type reactions using triflic acid include cracking of alkanes and alkylation of alkenes which are very important to the petroleum industry. These triflic acid derivative catalysts are very effective in isomerizing straight chain or slightly branched hydrocarbons that can increase the octane rating of a particular petroleum based fuel.
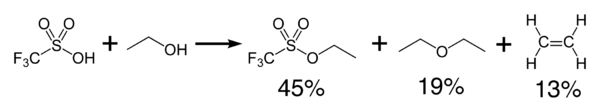
Triflic acid reacts exothermically with alcohols to produce ethers and olefins (1). It can be used as a catalyst for the condensation of alcohols and carboxylic acids.
References
- ^ Howells, R. D., McCown, J. D. "Trifluoromethanesulfonic Acid and Derivatives." Chemical Reviews, 1977. Vol. 77, pp 69 - 92; DOI: 10.1021/cr60305a005
- ^ Lakshminarayanapuram, R. S. "Trifluoromethanesulfonic Acid". Encyclopedia of Reagents for Organic Synthesis. 2001 John Wiley & Sons, Ltd. DOI: 10.1002/047084289X.rt246
See also
Dixon, N. E.; Lawrance, G. A.; Lay, P. A.; Sargeson, A. M.; Taube, H. "Trifluoromethanesulfonates and trifluoromethanesulfonato-O complexes" Inorganic Syntheses (1990), vol. 28, pp. 70-6.
