p53
p53, also known as Tumor protein P53, cellular tumor antigen p53 (UniProt name), or transformation-related protein 53 (TRP53) is a regulatory protein that is often mutated in human cancers. The p53 proteins (originally thought to be, and often spoken of as, a single protein) are crucial in vertebrates, where they prevent cancer formation.[5] As such, p53 has been described as "the guardian of the genome" because of its role in conserving stability by preventing genome mutation.[6] Hence TP53[note 1] is classified as a tumor suppressor gene.[7][8][9][10][11]
The TP53 gene is the most frequently mutated gene (>50%) in human cancer, indicating that the TP53 gene plays a crucial role in preventing cancer formation.[5] TP53 gene encodes proteins that bind to DNA and regulate gene expression to prevent mutations of the genome.[12] In addition to the full-length protein, the human TP53 gene encodes at least 12 protein isoforms.[13]
Gene
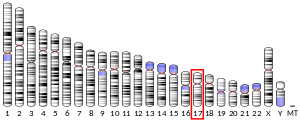
[edit]In humans, the TP53 gene is located on the short arm of chromosome 17 (17p13.1).[7][8][9][10] The gene spans 20 kb, with a non-coding exon 1 and a very long first intron of 10 kb, overlapping the Hp53int1 gene. The coding sequence contains five regions showing a high degree of conservation in vertebrates, predominantly in exons 2, 5, 6, 7 and 8, but the sequences found in invertebrates show only distant resemblance to mammalian TP53.[14] TP53 orthologs[15] have been identified in most mammals for which complete genome data are available.
Human TP53 gene
[edit]In humans, a common polymorphism involves the substitution of an arginine for a proline at codon position 72 of exon 4. Many studies have investigated a genetic link between this variation and cancer susceptibility; however, the results have been controversial. For instance, a meta-analysis from 2009 failed to show a link for cervical cancer.[16] A 2011 study found that the TP53 proline mutation did have a profound effect on pancreatic cancer risk among males.[17] A study of Arab women found that proline homozygosity at TP53 codon 72 is associated with a decreased risk for breast cancer.[18] One study suggested that TP53 codon 72 polymorphisms, MDM2 SNP309, and A2164G may collectively be associated with non-oropharyngeal cancer susceptibility and that MDM2 SNP309 in combination with TP53 codon 72 may accelerate the development of non-oropharyngeal cancer in women.[19] A 2011 study found that TP53 codon 72 polymorphism was associated with an increased risk of lung cancer.[20]
Meta-analyses from 2011 found no significant associations between TP53 codon 72 polymorphisms and both colorectal cancer risk[21] and endometrial cancer risk.[22] A 2011 study of a Brazilian birth cohort found an association between the non-mutant arginine TP53 and individuals without a family history of cancer.[23] Another 2011 study found that the p53 homozygous (Pro/Pro) genotype was associated with a significantly increased risk for renal cell carcinoma.[24]
Function
[edit]DNA damage and repair
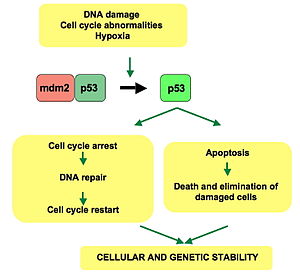
[edit]p53 plays a role in regulation or progression through the cell cycle, apoptosis, and genomic stability by means of several mechanisms:
- It can activate DNA repair proteins when DNA has sustained damage[25] Thus, it may be an important factor in aging.[26]
- It can arrest growth by holding the cell cycle at the G1/S regulation point on DNA damage recognition—if it holds the cell here for long enough, the DNA repair proteins will have time to fix the damage and the cell will be allowed to continue the cell cycle.
- It can initiate apoptosis (i.e., programmed cell death) if DNA damage proves to be irreparable.
- It is essential for the senescence response to short telomeres.
WAF1/CIP1 encodes for p21 and hundreds of other down-stream genes. p21 (WAF1) binds to the G1-S/CDK (CDK4/CDK6, CDK2, and CDK1) complexes (molecules important for the G1/S transition in the cell cycle) inhibiting their activity. [citation needed]
When p21(WAF1) is complexed with CDK2, the cell cannot continue to the next stage of cell division. A mutant p53 will no longer bind DNA in an effective way, and, as a consequence, the p21 protein will not be available to act as the "stop signal" for cell division.[27] Studies of human embryonic stem cells (hESCs) commonly describe the nonfunctional p53-p21 axis of the G1/S checkpoint pathway with subsequent relevance for cell cycle regulation and the DNA damage response (DDR). Importantly, p21 mRNA is clearly present and upregulated after the DDR in hESCs, but p21 protein is not detectable. In this cell type, p53 activates numerous microRNAs (like miR-302a, miR-302b, miR-302c, and miR-302d) that directly inhibit the p21 expression in hESCs. [citation needed]
The p21 protein binds directly to cyclin-CDK complexes that drive forward the cell cycle and inhibits their kinase activity, thereby causing cell cycle arrest to allow repair to take place. p21 can also mediate growth arrest associated with differentiation and a more permanent growth arrest associated with cellular senescence. The p21 gene contains several p53 response elements that mediate direct binding of the p53 protein, resulting in transcriptional activation of the gene encoding the p21 protein. [citation needed]

The p53 and RB1 pathways are linked via p14ARF, raising the possibility that the pathways may regulate each other.[28]
p53 expression can be stimulated by UV light, which also causes DNA damage. In this case, p53 can initiate events leading to tanning.[29][30]
Stem cells
[edit]Levels of p53 play an important role in the maintenance of stem cells throughout development and the rest of human life. [citation needed]
In human embryonic stem cells (hESCs)s, p53 is maintained at low inactive levels.[31] This is because activation of p53 leads to rapid differentiation of hESCs.[32] Studies have shown that knocking out p53 delays differentiation and that adding p53 causes spontaneous differentiation, showing how p53 promotes differentiation of hESCs and plays a key role in cell cycle as a differentiation regulator. When p53 becomes stabilized and activated in hESCs, it increases p21 to establish a longer G1. This typically leads to abolition of S-phase entry, which stops the cell cycle in G1, leading to differentiation. Work in mouse embryonic stem cells has recently shown however that the expression of P53 does not necessarily lead to differentiation.[33] p53 also activates miR-34a and miR-145, which then repress the hESCs pluripotency factors, further instigating differentiation.[31]
In adult stem cells, p53 regulation is important for maintenance of stemness in adult stem cell niches. Mechanical signals such as hypoxia affect levels of p53 in these niche cells through the hypoxia inducible factors, HIF-1α and HIF-2α. While HIF-1α stabilizes p53, HIF-2α suppresses it.[34] Suppression of p53 plays important roles in cancer stem cell phenotype, induced pluripotent stem cells and other stem cell roles and behaviors, such as blastema formation. Cells with decreased levels of p53 have been shown to reprogram into stem cells with a much greater efficiency than normal cells.[35][36] Papers suggest that the lack of cell cycle arrest and apoptosis gives more cells the chance to be reprogrammed. Decreased levels of p53 were also shown to be a crucial aspect of blastema formation in the legs of salamanders.[37] p53 regulation is very important in acting as a barrier between stem cells and a differentiated stem cell state, as well as a barrier between stem cells being functional and being cancerous.[38]
Other
[edit]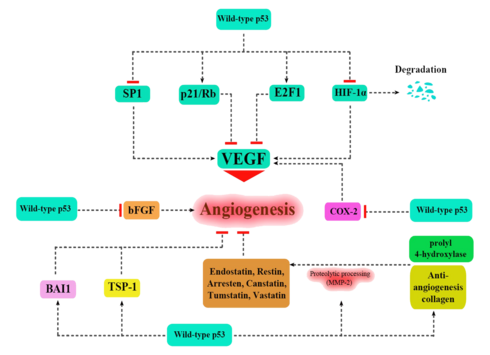
Apart from the cellular and molecular effects above, p53 has a tissue-level anticancer effect that works by inhibiting angiogenesis.[39] As tumors grow they need to recruit new blood vessels to supply them, and p53 inhibits that by (i) interfering with regulators of tumor hypoxia that also affect angiogenesis, such as HIF1 and HIF2, (ii) inhibiting the production of angiogenic promoting factors, and (iii) directly increasing the production of angiogenesis inhibitors, such as arresten.[40][41]
p53 by regulating Leukemia Inhibitory Factor has been shown to facilitate implantation in the mouse and possibly human reproduction.[42]
The immune response to infection also involves p53 and NF-κB. Checkpoint control of the cell cycle and of apoptosis by p53 is inhibited by some infections such as Mycoplasma bacteria,[43] raising the specter of oncogenic infection.
Regulation
[edit]p53 acts as a cellular stress sensor. It is normally kept at low levels by being constantly marked for degradation by the E3 ubiquitin ligase protein MDM2.[44] p53 is activated in response to myriad stressors – including DNA damage (induced by either UV, IR, or chemical agents such as hydrogen peroxide), oxidative stress,[45] osmotic shock, ribonucleotide depletion, viral lung infections[46] and deregulated oncogene expression. This activation is marked by two major events. First, the half-life of the p53 protein is increased drastically, leading to a quick accumulation of p53 in stressed cells. Second, a conformational change forces p53 to be activated as a transcription regulator in these cells. The critical event leading to the activation of p53 is the phosphorylation of its N-terminal domain. The N-terminal transcriptional activation domain contains a large number of phosphorylation sites and can be considered as the primary target for protein kinases transducing stress signals. [citation needed]
The protein kinases that are known to target this transcriptional activation domain of p53 can be roughly divided into two groups. A first group of protein kinases belongs to the MAPK family (JNK1-3, ERK1-2, p38 MAPK), which is known to respond to several types of stress, such as membrane damage, oxidative stress, osmotic shock, heat shock, etc. A second group of protein kinases (ATR, ATM, CHK1 and CHK2, DNA-PK, CAK, TP53RK) is implicated in the genome integrity checkpoint, a molecular cascade that detects and responds to several forms of DNA damage caused by genotoxic stress. Oncogenes also stimulate p53 activation, mediated by the protein p14ARF. [citation needed]
In unstressed cells, p53 levels are kept low through a continuous degradation of p53. A protein called Mdm2 (also called HDM2 in humans), binds to p53, preventing its action and transports it from the nucleus to the cytosol. Mdm2 also acts as an ubiquitin ligase and covalently attaches ubiquitin to p53 and thus marks p53 for degradation by the proteasome. However, ubiquitylation of p53 is reversible. On activation of p53, Mdm2 is also activated, setting up a feedback loop. p53 levels can show oscillations (or repeated pulses) in response to certain stresses, and these pulses can be important in determining whether the cells survive the stress, or die.[47]
MI-63 binds to MDM2, reactivating p53 in situations where p53's function has become inhibited.[48]
A ubiquitin specific protease, USP7 (or HAUSP), can cleave ubiquitin off p53, thereby protecting it from proteasome-dependent degradation via the ubiquitin ligase pathway. This is one means by which p53 is stabilized in response to oncogenic insults. USP42 has also been shown to deubiquitinate p53 and may be required for the ability of p53 to respond to stress.[49]
Recent research has shown that HAUSP is mainly localized in the nucleus, though a fraction of it can be found in the cytoplasm and mitochondria. Overexpression of HAUSP results in p53 stabilization. However, depletion of HAUSP does not result in a decrease in p53 levels but rather increases p53 levels due to the fact that HAUSP binds and deubiquitinates Mdm2. It has been shown that HAUSP is a better binding partner to Mdm2 than p53 in unstressed cells.
USP10, however, has been shown to be located in the cytoplasm in unstressed cells and deubiquitinates cytoplasmic p53, reversing Mdm2 ubiquitination. Following DNA damage, USP10 translocates to the nucleus and contributes to p53 stability. Also USP10 does not interact with Mdm2.[50]
Phosphorylation of the N-terminal end of p53 by the above-mentioned protein kinases disrupts Mdm2-binding. Other proteins, such as Pin1, are then recruited to p53 and induce a conformational change in p53, which prevents Mdm2-binding even more. Phosphorylation also allows for binding of transcriptional coactivators, like p300 and PCAF, which then acetylate the C-terminal end of p53, exposing the DNA binding domain of p53, allowing it to activate or repress specific genes. Deacetylase enzymes, such as Sirt1 and Sirt7, can deacetylate p53, leading to an inhibition of apoptosis.[51] Some oncogenes can also stimulate the transcription of proteins that bind to MDM2 and inhibit its activity. [citation needed]
Epigenetic marks like histone methylation can also regulate p53, for example, p53 interacts directly with a repressive Trim24 cofactor that binds histones in regions of the genome that are epigenetically repressed.[52] Trim24 prevents p53 from activating its targets, but only in these regions, effectively giving p53 the ability to 'read out' the histone profile at key target genes and act in a gene-specific manner. [citation needed]
Role in disease
[edit]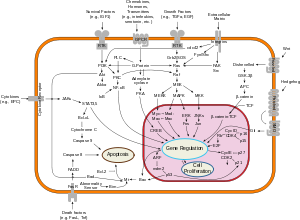
If the TP53 gene is damaged, tumor suppression is severely compromised. People who inherit only one functional copy of the TP53 gene will most likely develop tumors in early adulthood, a disorder known as Li–Fraumeni syndrome. [citation needed]
The TP53 gene can also be modified by mutagens (chemicals, radiation, or viruses), increasing the likelihood for uncontrolled cell division. More than 50 percent of human tumors contain a mutation or deletion of the TP53 gene.[53] Loss of p53 creates genomic instability that most often results in an aneuploidy phenotype.[54]
Increasing the amount of p53 may seem a solution for treatment of tumors or prevention of their spreading. This, however, is not a usable method of treatment, since it can cause premature aging.[55] Restoring endogenous normal p53 function holds some promise. Research has shown that this restoration can lead to regression of certain cancer cells without damaging other cells in the process. The ways by which tumor regression occurs depends mainly on the tumor type. For example, restoration of endogenous p53 function in lymphomas may induce apoptosis, while cell growth may be reduced to normal levels. Thus, pharmacological reactivation of p53 presents itself as a viable cancer treatment option.[56][57] The first commercial gene therapy, Gendicine, was approved in China in 2003 for the treatment of head and neck squamous cell carcinoma. It delivers a functional copy of the p53 gene using an engineered adenovirus.[58]
Certain pathogens can also affect the p53 protein that the TP53 gene expresses. One such example, human papillomavirus (HPV), encodes a protein, E6, which binds to the p53 protein and inactivates it. This mechanism, in synergy with the inactivation of the cell cycle regulator pRb by the HPV protein E7, allows for repeated cell division manifested clinically as warts. Certain HPV types, in particular types 16 and 18, can also lead to progression from a benign wart to low or high-grade cervical dysplasia, which are reversible forms of precancerous lesions. Persistent infection of the cervix over the years can cause irreversible changes leading to carcinoma in situ and eventually invasive cervical cancer. This results from the effects of HPV genes, particularly those encoding E6 and E7, which are the two viral oncoproteins that are preferentially retained and expressed in cervical cancers by integration of the viral DNA into the host genome.[59]
The p53 protein is continually produced and degraded in cells of healthy people, resulting in damped oscillation (see a stochastic model of this process in [60]). The degradation of the p53 protein is associated with binding of MDM2. In a negative feedback loop, MDM2 itself is induced by the p53 protein. Mutant p53 proteins often fail to induce MDM2, causing p53 to accumulate at very high levels. Moreover, the mutant p53 protein itself can inhibit normal p53 protein levels. In some cases, single missense mutations in p53 have been shown to disrupt p53 stability and function.[61]
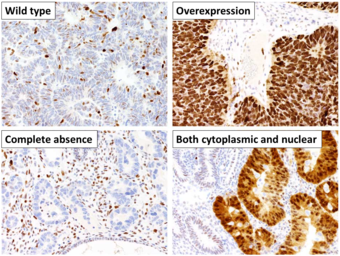 This image shows different patterns of p53 expression in endometrial cancers on chromogenic immunohistochemistry, whereof all except wild-type are variably termed abnormal/aberrant/mutation-type and are strongly predictive of an underlying TP53 mutation:[62]
|
Suppression of p53 in human breast cancer cells is shown to lead to increased CXCR5 chemokine receptor gene expression and activated cell migration in response to chemokine CXCL13.[65]
One study found that p53 and Myc proteins were key to the survival of Chronic Myeloid Leukaemia (CML) cells. Targeting p53 and Myc proteins with drugs gave positive results on mice with CML.[66][67]
Experimental analysis of p53 mutations
[edit]Most p53 mutations are detected by DNA sequencing. However, it is known that single missense mutations can have a large spectrum from rather mild to very severe functional effects.[61]

The large spectrum of cancer phenotypes due to mutations in the TP53 gene is also supported by the fact that different isoforms of p53 proteins have different cellular mechanisms for prevention against cancer. Mutations in TP53 can give rise to different isoforms, preventing their overall functionality in different cellular mechanisms and thereby extending the cancer phenotype from mild to severe. Recent studies show that p53 isoforms are differentially expressed in different human tissues, and the loss-of-function or gain-of-function mutations within the isoforms can cause tissue-specific cancer or provide cancer stem cell potential in different tissues.[11][69][70][71] TP53 mutation also hits energy metabolism and increases glycolysis in breast cancer cells.[72]
The dynamics of p53 proteins, along with its antagonist Mdm2, indicate that the levels of p53, in units of concentration, oscillate as a function of time. This "damped" oscillation is both clinically documented [73] and mathematically modelled.[74][75] Mathematical models also indicate that the p53 concentration oscillates much faster once teratogens, such as double-stranded breaks (DSB) or UV radiation, are introduced to the system. This supports and models the current understanding of p53 dynamics, where DNA damage induces p53 activation (see p53 regulation for more information). Current models can also be useful for modelling the mutations in p53 isoforms and their effects on p53 oscillation, thereby promoting de novo tissue-specific pharmacological drug discovery. [citation needed]
Discovery
[edit]p53 was identified in 1979 by Lionel Crawford, David P. Lane, Arnold Levine, and Lloyd Old, working at Imperial Cancer Research Fund (UK) Princeton University/UMDNJ (Cancer Institute of New Jersey), and Memorial Sloan Kettering Cancer Center, respectively. It had been hypothesized to exist before as the target of the SV40 virus, a strain that induced development of tumors. The name p53 was given in 1979 describing the apparent molecular mass. [citation needed]
The TP53 gene from the mouse was first cloned by Peter Chumakov of The Academy of Sciences of the USSR in 1982,[76] and independently in 1983 by Moshe Oren in collaboration with David Givol (Weizmann Institute of Science).[77][78] The human TP53 gene was cloned in 1984[7] and the full length clone in 1985.[79]
It was initially presumed to be an oncogene due to the use of mutated cDNA following purification of tumor cell mRNA. Its role as a tumor suppressor gene was revealed in 1989 by Bert Vogelstein at the Johns Hopkins School of Medicine and Arnold Levine at Princeton University.[80][81] p53 went on to be identified as a transcription factor by Guillermina Lozano working at MD Anderson Cancer Center.[82]
Warren Maltzman, of the Waksman Institute of Rutgers University first demonstrated that TP53 was responsive to DNA damage in the form of ultraviolet radiation.[83] In a series of publications in 1991–92, Michael Kastan of Johns Hopkins University, reported that TP53 was a critical part of a signal transduction pathway that helped cells respond to DNA damage.[84]
In 1993, p53 was voted molecule of the year by Science magazine.[85]
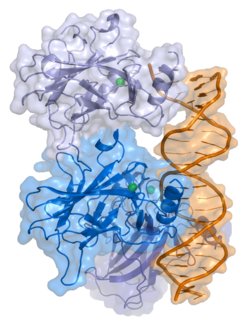
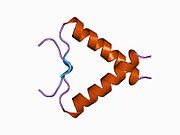
Structure
[edit]
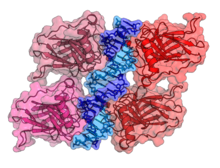
p53 has seven domains:
- an acidic N-terminus transcription-activation domain (TAD), also known as activation domain 1 (AD1), which activates transcription factors. The N-terminus contains two complementary transcriptional activation domains, with a major one at residues 1–42 and a minor one at residues 55–75, specifically involved in the regulation of several pro-apoptotic genes.[86]
- activation domain 2 (AD2) important for apoptotic activity: residues 43–63.
- proline rich domain important for the apoptotic activity of p53 by nuclear exportation via MAPK: residues 64–92.
- central DNA-binding core domain (DBD). Contains one zinc atom and several arginine amino acids: residues 102–292. This region is responsible for binding the p53 co-repressor LMO3.[87]
- Nuclear Localization Signaling (NLS) domain, residues 316–325.
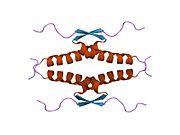
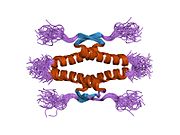
- homo-oligomerisation domain (OD): residues 307–355. Tetramerization is essential for the activity of p53 in vivo.
- C-terminal involved in downregulation of DNA binding of the central domain: residues 356–393.[88]
Mutations that deactivate p53 in cancer usually occur in the DBD. Most of these mutations destroy the ability of the protein to bind to its target DNA sequences, and thus prevents transcriptional activation of these genes. As such, mutations in the DBD are recessive loss-of-function mutations. Molecules of p53 with mutations in the OD dimerise with wild-type p53, and prevent them from activating transcription. Therefore, OD mutations have a dominant negative effect on the function of p53.
Wild-type p53 is a labile protein, comprising folded and unstructured regions that function in a synergistic manner.[89]
SDS-PAGE analysis indicates that p53 is a 53-kilodalton (kDa) protein. However, the actual mass of the full-length p53 protein (p53α) based on the sum of masses of the amino acid residues is only 43.7 kDa. This difference is due to the high number of proline residues in the protein, which slow its migration on SDS-PAGE, thus making it appear heavier than it actually is.[90]
Isoforms
[edit]As with 95% of human genes, TP53 encodes more than one protein. All these p53 proteins are called the p53 isoforms.[5] These proteins range in size from 3.5 to 43.7 kDa. Several isoforms were discovered in 2005, and so far 12 human p53 isoforms have been identified (p53α, p53β, p53γ, ∆40p53α, ∆40p53β, ∆40p53γ, ∆133p53α, ∆133p53β, ∆133p53γ, ∆160p53α, ∆160p53β, ∆160p53γ). Furthermore, p53 isoforms are expressed in a tissue dependent manner and p53α is never expressed alone.[11]
The full length p53 isoform proteins can be subdivided into different protein domains. Starting from the N-terminus, there are first the amino-terminal transcription-activation domains (TAD 1, TAD 2), which are needed to induce a subset of p53 target genes. This domain is followed by the proline rich domain (PXXP), whereby the motif PXXP is repeated (P is a proline and X can be any amino acid). It is required among others for p53 mediated apoptosis.[91] Some isoforms lack the proline rich domain, such as Δ133p53β,γ and Δ160p53α,β,γ; hence some isoforms of p53 are not mediating apoptosis, emphasizing the diversifying roles of the TP53 gene.[69] Afterwards there is the DNA binding domain (DBD), which enables the proteins to sequence specific binding. The C-terminus domain completes the protein. It includes the nuclear localization signal (NLS), the nuclear export signal (NES) and the oligomerisation domain (OD). The NLS and NES are responsible for the subcellular regulation of p53. Through the OD, p53 can form a tetramer and then bind to DNA. Among the isoforms, some domains can be missing, but all of them share most of the highly conserved DNA-binding domain. [citation needed]
The isoforms are formed by different mechanisms. The beta and the gamma isoforms are generated by multiple splicing of intron 9, which leads to a different C-terminus. Furthermore, the usage of an internal promoter in intron 4 causes the ∆133 and ∆160 isoforms, which lack the TAD domain and a part of the DBD. Moreover, alternative initiation of translation at codon 40 or 160 bear the ∆40p53 and ∆160p53 isoforms.[11]
Due to the isoformic nature of p53 proteins, there have been several sources of evidence showing that mutations within the TP53 gene giving rise to mutated isoforms are causative agents of various cancer phenotypes, from mild to severe, due to single mutation in the TP53 gene (refer to section Experimental analysis of p53 mutations for more details).
Interactions
[edit]p53 has been shown to interact with:
- AIMP2,[92]
- ANKRD2,[93]
- APTX,[94]
- ATM,[95][96][97][98][99]
- ATR,[95][96]
- ATF3,[100][101]
- AURKA,[102]
- BAK1,[103]
- BARD1,[104]
- BLM,[105][106][107][108]
- BRCA1,[104][109][110][111][112]
- BRCA2,[104][113]
- BRCC3,[104]
- BRE,[104]
- CEBPZ,[114]
- CDC14A,[115]
- Cdk1,[116][117]
- CFLAR,[118]
- CHEK1,[105][119][120]
- CCNG1,[121]
- CREBBP,[122][123][124]
- CREB1,[124]
- Cyclin H,[125]
- CDK7,[125][126]
- DNA-PKcs,[96][119][127]
- E4F1,[128][129]
- EFEMP2,[130]
- EIF2AK2,[131]
- ELL,[132]
- EP300,[123][133][134][135]
- ERCC6,[136][137]
- GNL3,[138]
- GPS2,[139]
- GSK3B,[140]
- HSP90AA1,[141][142][143]
- HIF1A,[144][145][146][147]
- HIPK1,[148]
- HIPK2,[149][150]
- HMGB1,[151][152]
- HSPA9,[153]
- Huntingtin,[154]
- ING1,[155][156]
- ING4,[157][158]
- ING5,[157]
- IκBα,[159]
- KPNB1,[141]
- LMO3,[87]
- Mdm2,[122][160][161][162]
- MDM4,[163][164]
- MED1,[165][166]
- MAPK9,[167][168]
- MNAT1,[126]
- NDN,[169]
- NCL,[170]
- NUMB,[171]
- NF-κB,[172]
- P16,[128][162][173]
- PARC,[174]
- PARP1,[94][175]
- PIAS1,[130][176]
- CDC14B,[115]
- PIN1,[177][178]
- PLAGL1,[179]
- PLK3,[180][181]
- PRKRA,[182]
- PHB,[183]
- PML,[160][184][185]
- PSME3,[186]
- PTEN,[161]
- PTK2,[187]
- PTTG1,[188]
- RAD51,[104][189][190]
- RCHY1,[191][192]
- RELA,[172]
- Reprimo[citation needed]
- RPA1,[193][194]
- RPL11,[173]
- S100B,[195]
- SUMO1,[196][197]
- SMARCA4,[198]
- SMARCB1,[198]
- SMN1,[199]
- STAT3,[172]
- TBP,[200][201]
- TFAP2A,[202]
- TFDP1,[203]
- TIGAR,[204]
- TOP1,[205][206]
- TOP2A,[207]
- TP53BP1,[105][208][209][210][211][212][213]
- TP53BP2,[213][214]
- TOP2B,[207]
- TP53INP1,[215][216]
- TSG101,[217]
- UBE2A,[218]
- UBE2I,[130][196][219][220]
- UBC,[92][186][197][221][222][223][224][225]
- USP7,[226]
- USP10,[50]
- WRN,[108][227]
- WWOX,[228]
- XPB,[136]
- YBX1,[93][229]
- YPEL3,[230]
- YWHAZ,[231]
- Zif268,[232]
- ZNF148,[233]
- SIRT1,[234]
- circRNA_014511.[235]
See also
[edit]- Pifithrin, an inhibitor of P53
Notes
[edit]References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000141510 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000059552 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c Surget S, Khoury MP, Bourdon JC (December 2013). "Uncovering the role of p53 splice variants in human malignancy: a clinical perspective". OncoTargets and Therapy. 7: 57–68. doi:10.2147/OTT.S53876. PMC 3872270. PMID 24379683.
- ^ Toufektchan E, Toledo F (May 2018). "The Guardian of the Genome Revisited: p53 Downregulates Genes Required for Telomere Maintenance, DNA Repair, and Centromere Structure". Cancers. 10 (5): 135. doi:10.3390/cancers10050135. PMC 5977108. PMID 29734785.
- ^ a b c Matlashewski G, Lamb P, Pim D, et al. (December 1984). "Isolation and characterization of a human p53 cDNA clone: expression of the human p53 gene". The EMBO Journal. 3 (13): 3257–62. doi:10.1002/j.1460-2075.1984.tb02287.x. PMC 557846. PMID 6396087.
- ^ a b Isobe M, Emanuel BS, Givol D, et al. (1986). "Localization of gene for human p53 tumour antigen to band 17p13". Nature. 320 (6057): 84–5. Bibcode:1986Natur.320...84I. doi:10.1038/320084a0. PMID 3456488. S2CID 4310476.
- ^ a b Kern SE, Kinzler KW, Bruskin A, et al. (June 1991). "Identification of p53 as a sequence-specific DNA-binding protein". Science. 252 (5013): 1708–11. Bibcode:1991Sci...252.1708K. doi:10.1126/science.2047879. PMID 2047879. S2CID 19647885.
- ^ a b McBride OW, Merry D, Givol D (January 1986). "The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13)". Proceedings of the National Academy of Sciences of the United States of America. 83 (1): 130–4. Bibcode:1986PNAS...83..130M. doi:10.1073/pnas.83.1.130. PMC 322805. PMID 3001719.
- ^ a b c d Bourdon JC, Fernandes K, Murray-Zmijewski F, et al. (September 2005). "p53 isoforms can regulate p53 transcriptional activity". Genes & Development. 19 (18): 2122–37. doi:10.1101/gad.1339905. PMC 1221884. PMID 16131611.
- ^ Levine AJ, Lane DP, eds. (2010). The p53 family. Cold Spring Harbor Perspectives in Biology. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-830-0.
- ^ Khoury MP, Bourdon JC (April 2011). "p53 Isoforms: An Intracellular Microprocessor?". Genes Cancer. 2 (4): 453–65. doi:10.1177/1947601911408893. PMC 3135639. PMID 21779513.
- ^ May P, May E (December 1999). "Twenty years of p53 research: structural and functional aspects of the p53 protein". Oncogene. 18 (53): 7621–36. doi:10.1038/sj.onc.1203285. PMID 10618702.
- ^ "OrthoMaM phylogenetic marker: TP53 coding sequence". Archived from the original on 2018-03-17. Retrieved 2009-12-02.
- ^ Klug SJ, Ressing M, Koenig J, et al. (August 2009). "TP53 codon 72 polymorphism and cervical cancer: a pooled analysis of individual data from 49 studies". The Lancet. Oncology. 10 (8): 772–84. doi:10.1016/S1470-2045(09)70187-1. PMID 19625214.
- ^ Sonoyama T, Sakai A, Mita Y, et al. (2011). "TP53 codon 72 polymorphism is associated with pancreatic cancer risk in males, smokers and drinkers". Molecular Medicine Reports. 4 (3): 489–95. doi:10.3892/mmr.2011.449. PMID 21468597.
- ^ Alawadi S, Ghabreau L, Alsaleh M, et al. (September 2011). "P53 gene polymorphisms and breast cancer risk in Arab women". Medical Oncology. 28 (3): 709–15. doi:10.1007/s12032-010-9505-4. PMID 20443084. S2CID 207372095.
- ^ Yu H, Huang YJ, Liu Z, et al. (September 2011). "Effects of MDM2 promoter polymorphisms and p53 codon 72 polymorphism on risk and age at onset of squamous cell carcinoma of the head and neck". Molecular Carcinogenesis. 50 (9): 697–706. doi:10.1002/mc.20806. PMC 3142329. PMID 21656578.
- ^ Piao JM, Kim HN, Song HR, et al. (September 2011). "p53 codon 72 polymorphism and the risk of lung cancer in a Korean population". Lung Cancer. 73 (3): 264–7. doi:10.1016/j.lungcan.2010.12.017. PMID 21316118.
- ^ Wang JJ, Zheng Y, Sun L, et al. (November 2011). "TP53 codon 72 polymorphism and colorectal cancer susceptibility: a meta-analysis". Molecular Biology Reports. 38 (8): 4847–53. doi:10.1007/s11033-010-0619-8. PMID 21140221. S2CID 11730631.
- ^ Jiang DK, Yao L, Ren WH, et al. (December 2011). "TP53 Arg72Pro polymorphism and endometrial cancer risk: a meta-analysis". Medical Oncology. 28 (4): 1129–35. doi:10.1007/s12032-010-9597-x. PMID 20552298. S2CID 32990396.
- ^ Thurow HS, Haack R, Hartwig FP, et al. (December 2011). "TP53 gene polymorphism: importance to cancer, ethnicity and birth weight in a Brazilian cohort". Journal of Biosciences. 36 (5): 823–31. doi:10.1007/s12038-011-9147-5. PMID 22116280. S2CID 23027087.
- ^ Huang CY, Su CT, Chu JS, et al. (December 2011). "The polymorphisms of P53 codon 72 and MDM2 SNP309 and renal cell carcinoma risk in a low arsenic exposure area". Toxicology and Applied Pharmacology. 257 (3): 349–55. Bibcode:2011ToxAP.257..349H. doi:10.1016/j.taap.2011.09.018. PMID 21982800.
- ^ a b Janic A, Abad E, Amelio I (2024-02-20). "Decoding p53 tumor suppression: a crosstalk between genomic stability and epigenetic control?". Cell Death & Differentiation: 1–8. doi:10.1038/s41418-024-01259-9. ISSN 1476-5403.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Gilbert SF. Developmental Biology, 10th ed. Sunderland, MA USA: Sinauer Associates, Inc. Publishers. p. 588.
- ^ National Center for Biotechnology Information (1998). "Skin and Connective Tissue". Genes and Disease. United States National Institutes of Health. Retrieved 2008-05-28.
- ^ Bates S, Phillips AC, Clark PA, et al. (September 1998). "p14ARF links the tumour suppressors RB and p53". Nature. 395 (6698): 124–5. Bibcode:1998Natur.395..124B. doi:10.1038/25867. PMID 9744267. S2CID 4355786.
- ^ "Genome's guardian gets a tan started". New Scientist. March 17, 2007. Retrieved 2007-03-29.
- ^ Cui R, Widlund HR, Feige E, et al. (March 2007). "Central role of p53 in the suntan response and pathologic hyperpigmentation". Cell. 128 (5): 853–64. doi:10.1016/j.cell.2006.12.045. PMID 17350573.
- ^ a b Jain AK, Allton K, Iacovino M, et al. (2012). "p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells". PLOS Biology. 10 (2): e1001268. doi:10.1371/journal.pbio.1001268. PMC 3289600. PMID 22389628.
- ^ Maimets T, Neganova I, Armstrong L, et al. (September 2008). "Activation of p53 by nutlin leads to rapid differentiation of human embryonic stem cells". Oncogene. 27 (40): 5277–87. doi:10.1038/onc.2008.166. PMID 18521083.
- ^ ter Huurne M, Peng T, Yi G, et al. (February 2020). "Critical role for P53 in regulating the cell cycle of ground state embryonic stem cells". Stem Cell Reports. 14 (2): 175–183. doi:10.1016/j.stemcr.2020.01.001. PMC 7013234. PMID 32004494.
- ^ Das B, Bayat-Mokhtari R, Tsui M, et al. (August 2012). "HIF-2α suppresses p53 to enhance the stemness and regenerative potential of human embryonic stem cells". Stem Cells. 30 (8): 1685–95. doi:10.1002/stem.1142. PMC 3584519. PMID 22689594.
- ^ Lake BB, Fink J, Klemetsaune L, et al. (May 2012). "Context-dependent enhancement of induced pluripotent stem cell reprogramming by silencing Puma". Stem Cells. 30 (5): 888–97. doi:10.1002/stem.1054. PMC 3531606. PMID 22311782.
- ^ Marión RM, Strati K, Li H, et al. (August 2009). "A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity". Nature. 460 (7259): 1149–53. Bibcode:2009Natur.460.1149M. doi:10.1038/nature08287. PMC 3624089. PMID 19668189.
- ^ Yun MH, Gates PB, Brockes JP (October 2013). "Regulation of p53 is critical for vertebrate limb regeneration". Proceedings of the National Academy of Sciences of the United States of America. 110 (43): 17392–7. Bibcode:2013PNAS..11017392Y. doi:10.1073/pnas.1310519110. PMC 3808590. PMID 24101460.
- ^ Aloni-Grinstein R, Shetzer Y, Kaufman T, et al. (August 2014). "p53: the barrier to cancer stem cell formation". FEBS Letters. 588 (16): 2580–9. Bibcode:2014FEBSL.588.2580A. doi:10.1016/j.febslet.2014.02.011. PMID 24560790. S2CID 37901173.
- ^ a b Babaei G, Aliarab A, Asghari Vostakolaei M, et al. (November 2021). "Crosslink between p53 and metastasis: focus on epithelial-mesenchymal transition, cancer stem cell, angiogenesis, autophagy, and anoikis". Molecular Biology Reports. 48 (11): 7545–7557. doi:10.1007/s11033-021-06706-1. PMID 34519942. S2CID 237506513.
- ^ Teodoro JG, Evans SK, Green MR (November 2007). "Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome". Journal of Molecular Medicine (Review). 85 (11): 1175–1186. doi:10.1007/s00109-007-0221-2. PMID 17589818. S2CID 10094554.
- ^ Assadian S, El-Assaad W, Wang XQ, et al. (March 2012). "p53 inhibits angiogenesis by inducing the production of Arresten". Cancer Research. 72 (5): 1270–1279. doi:10.1158/0008-5472.CAN-11-2348. PMID 22253229.
- ^ Hu W, Feng Z, Teresky AK, et al. (November 2007). "p53 regulates maternal reproduction through LIF". Nature. 450 (7170): 721–4. Bibcode:2007Natur.450..721H. doi:10.1038/nature05993. PMID 18046411. S2CID 4357527.
- ^ Borchsenius SN, Daks A, Fedorova O, et al. (January 2018). "Effects of mycoplasma infection on the host organism response via p53/NF-κB signaling". Journal of Cellular Physiology. 234 (1): 171–180. doi:10.1002/jcp.26781. PMID 30146800.
- ^ Bykov VJ, Eriksson SE, Bianchi J, et al. (February 2018). "Targeting mutant p53 for efficient cancer therapy". Nature Reviews. Cancer. 18 (2): 89–102. doi:10.1038/nrc.2017.109. PMID 29242642. S2CID 4552678.
- ^ Han ES, Muller FL, Pérez VI, et al. (June 2008). "The in vivo gene expression signature of oxidative stress". Physiological Genomics. 34 (1): 112–126. doi:10.1152/physiolgenomics.00239.2007. PMC 2532791. PMID 18445702.
- ^ Grajales-Reyes GE, Colonna M (August 2020). "Interferon responses in viral pneumonias". Science. 369 (6504): 626–627. Bibcode:2020Sci...369..626G. doi:10.1126/science.abd2208. PMID 32764056.
- ^ Purvis JE, Karhohs KW, Mock C, et al. (June 2012). "p53 dynamics control cell fate". Science. 336 (6087): 1440–1444. Bibcode:2012Sci...336.1440P. doi:10.1126/science.1218351. PMC 4162876. PMID 22700930.
- ^ Canner JA, Sobo M, Ball S, et al. (September 2009). "MI-63: a novel small-molecule inhibitor targets MDM2 and induces apoptosis in embryonal and alveolar rhabdomyosarcoma cells with wild-type p53". British Journal of Cancer. 101 (5): 774–81. doi:10.1038/sj.bjc.6605199. PMC 2736841. PMID 19707204.
- ^ Hock AK, Vigneron AM, Carter S, et al. (November 2011). "Regulation of p53 stability and function by the deubiquitinating enzyme USP42". The EMBO Journal. 30 (24): 4921–30. doi:10.1038/emboj.2011.419. PMC 3243628. PMID 22085928.
- ^ a b Yuan J, Luo K, Zhang L, et al. (February 2010). "USP10 Regulates p53 Localization and Stability by Deubiquitinating p53". Cell. 140 (3): 384–396. doi:10.1016/j.cell.2009.12.032. PMC 2820153. PMID 20096447.
- ^ Vakhrusheva O, Smolka C, Gajawada P, et al. (March 2008). "Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice". Circulation Research. 102 (6): 703–10. doi:10.1161/CIRCRESAHA.107.164558. PMID 18239138.
- ^ Isbel L, Iskar M, Durdu S, et al. (June 2023). "Readout of histone methylation by Trim24 locally restricts chromatin opening by p53". Nature Structural & Molecular Biology. 30 (7): 948–57. doi:10.1038/s41594-023-01021-8. hdl:2440/139184. PMC 10352137. PMID 37386214.
- ^ Hollstein M, Sidransky D, Vogelstein B, et al. (July 1991). "p53 mutations in human cancers". Science. 253 (5015): 49–53. Bibcode:1991Sci...253...49H. doi:10.1126/science.1905840. PMID 1905840. S2CID 38527914.
- ^ Schmitt CA, Fridman JS, Yang M, et al. (April 2002). "Dissecting p53 tumor suppressor functions in vivo". Cancer Cell. 1 (3): 289–98. doi:10.1016/S1535-6108(02)00047-8. PMID 12086865.
- ^ Tyner SD, Venkatachalam S, Choi J, et al. (January 2002). "p53 mutant mice that display early ageing-associated phenotypes". Nature. 415 (6867): 45–53. Bibcode:2002Natur.415...45T. doi:10.1038/415045a. PMID 11780111. S2CID 749047.
- ^ Ventura A, Kirsch DG, McLaughlin ME, et al. (February 2007). "Restoration of p53 function leads to tumour regression in vivo". Nature. 445 (7128): 661–5. doi:10.1038/nature05541. PMID 17251932. S2CID 4373520.
- ^ Herce HD, Deng W, Helma J, et al. (2013). "Visualization and targeted disruption of protein interactions in living cells". Nature Communications. 4: 2660. Bibcode:2013NatCo...4.2660H. doi:10.1038/ncomms3660. PMC 3826628. PMID 24154492.
- ^ Pearson S, Jia H, Kandachi K (January 2004). "China approves first gene therapy". Nature Biotechnology. 22 (1): 3–4. doi:10.1038/nbt0104-3. PMC 7097065. PMID 14704685.
- ^ Angeletti PC, Zhang L, Wood C (2008). "The Viral Etiology of AIDS-Associated Malignancies". HIV-1: Molecular Biology and Pathogenesis. Advances in Pharmacology. Vol. 56. pp. 509–57. doi:10.1016/S1054-3589(07)56016-3. ISBN 978-0-12-373601-7. PMC 2149907. PMID 18086422.
- ^ Ribeiro AS, Charlebois DA, Lloyd-Price J (December 2007). "CellLine, a stochastic cell lineage simulator". Bioinformatics. 23 (24): 3409–3411. doi:10.1093/bioinformatics/btm491. PMID 17928303.
- ^ a b Bullock AN, Henckel J, DeDecker BS, et al. (December 1997). "Thermodynamic stability of wild-type and mutant p53 core domain". Proceedings of the National Academy of Sciences of the United States of America. 94 (26): 14338–42. Bibcode:1997PNAS...9414338B. doi:10.1073/pnas.94.26.14338. PMC 24967. PMID 9405613.
- ^ Köbel M, Ronnett BM, Singh N, et al. (January 2019). "Interpretation of P53 Immunohistochemistry in Endometrial Carcinomas: Toward Increased Reproducibility". International Journal of Gynecological Pathology. 38 (Suppl 1): S123 – S131. doi:10.1097/PGP.0000000000000488. PMC 6127005. PMID 29517499.
 This article incorporates text available under the CC BY 4.0 license.
This article incorporates text available under the CC BY 4.0 license.
- ^ Image is taken from following source, with some modification by Mikael Häggström, MD:
- Schallenberg S, Plage H, Hofbauer S, et al. (2023). "Altered p53/p16 expression is linked to urothelial carcinoma progression but largely unrelated to prognosis in muscle-invasive tumors". Acta Oncol. 62 (12): 1880–1889. doi:10.1080/0284186X.2023.2277344. PMID 37938166. - ^ Source for role in distinguishing PUNLMP from low-grade carcinoma:
- Kalantari MR, Ahmadnia H (2007). "P53 overexpression in bladder urothelial neoplasms: new aspect of World Health Organization/International Society of Urological Pathology classification". Urol J. 4 (4): 230–3. PMID 18270948. - ^ Mitkin NA, Hook CD, Schwartz AM, et al. (March 2015). "p53-dependent expression of CXCR5 chemokine receptor in MCF-7 breast cancer cells". Scientific Reports. 5 (5): 9330. Bibcode:2015NatSR...5E9330M. doi:10.1038/srep09330. PMC 4365401. PMID 25786345.
- ^ Abraham SA, Hopcroft LE, Carrick E, et al. (June 2016). "Dual targeting of p53 and c-MYC selectively eliminates leukaemic stem cells". Nature. 534 (7607): 341–6. Bibcode:2016Natur.534..341A. doi:10.1038/nature18288. PMC 4913876. PMID 27281222.
- ^ "Scientists identify drugs to target 'Achilles heel' of Chronic Myeloid Leukaemia cells". myScience. 2016-06-08. Retrieved 2016-06-09.
- ^ a b Butera A, Amelio I (July 2024). "Deciphering the significance of p53 mutant proteins". Trends in Cell Biology. doi:10.1016/j.tcb.2024.06.003. PMID 38960851.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ a b Khoury MP, Bourdon JC (April 2011). "p53 Isoforms: An Intracellular Microprocessor?". Genes & Cancer. 2 (4): 453–65. doi:10.1177/1947601911408893. PMC 3135639. PMID 21779513.
- ^ Avery-Kiejda KA, Morten B, Wong-Brown MW, et al. (March 2014). "The relative mRNA expression of p53 isoforms in breast cancer is associated with clinical features and outcome". Carcinogenesis. 35 (3): 586–96. doi:10.1093/carcin/bgt411. PMID 24336193.
- ^ Arsic N, Gadea G, Lagerqvist EL, et al. (April 2015). "The p53 isoform Δ133p53β promotes cancer stem cell potential". Stem Cell Reports. 4 (4): 531–40. doi:10.1016/j.stemcr.2015.02.001. PMC 4400643. PMID 25754205.
- ^ Harami-Papp H, Pongor LS, Munkácsy G, et al. (October 2016). "TP53 mutation hits energy metabolism and increases glycolysis in breast cancer". Oncotarget. 7 (41): 67183–67195. doi:10.18632/oncotarget.11594. PMC 5341867. PMID 27582538.
- ^ Geva-Zatorsky N, Rosenfeld N, Itzkovitz S, et al. (June 2006). "Oscillations and variability in the p53 system". Molecular Systems Biology. 2: 2006.0033. doi:10.1038/msb4100068. PMC 1681500. PMID 16773083.
- ^ Proctor CJ, Gray DA (August 2008). "Explaining oscillations and variability in the p53-Mdm2 system". BMC Systems Biology. 2 (75): 75. doi:10.1186/1752-0509-2-75. PMC 2553322. PMID 18706112.
- ^ Chong KH, Samarasinghe S, Kulasiri D (December 2013). "Mathematical modelling of p53 basal dynamics and DNA damage response". C-fACS. 259 (20th International Congress on Mathematical Modelling and Simulation): 670–6. doi:10.1016/j.mbs.2014.10.010. PMID 25433195.
- ^ Chumakov PM, Iotsova VS, Georgiev GP (1982). "[Isolation of a plasmid clone containing the mRNA sequence for mouse nonviral T-antigen]". Doklady Akademii Nauk SSSR (in Russian). 267 (5): 1272–5. PMID 6295732.
- ^ Oren M, Levine AJ (January 1983). "Molecular cloning of a cDNA specific for the murine p53 cellular tumor antigen". Proceedings of the National Academy of Sciences of the United States of America. 80 (1): 56–9. Bibcode:1983PNAS...80...56O. doi:10.1073/pnas.80.1.56. PMC 393308. PMID 6296874.
- ^ Zakut-Houri R, Oren M, Bienz B, et al. (1983). "A single gene and a pseudogene for the cellular tumour antigen p53". Nature. 306 (5943): 594–7. Bibcode:1983Natur.306..594Z. doi:10.1038/306594a0. PMID 6646235. S2CID 4325094.
- ^ Zakut-Houri R, Bienz-Tadmor B, Givol D, et al. (May 1985). "Human p53 cellular tumor antigen: cDNA sequence and expression in COS cells". The EMBO Journal. 4 (5): 1251–5. doi:10.1002/j.1460-2075.1985.tb03768.x. PMC 554332. PMID 4006916.
- ^ Baker SJ, Fearon ER, Nigro JM, et al. (April 1989). "Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas". Science. 244 (4901): 217–21. Bibcode:1989Sci...244..217B. doi:10.1126/science.2649981. PMID 2649981.
- ^ Finlay CA, Hinds PW, Levine AJ (June 1989). "The p53 proto-oncogene can act as a suppressor of transformation". Cell. 57 (7): 1083–93. doi:10.1016/0092-8674(89)90045-7. PMID 2525423.
- ^ Raycroft L, Wu HY, Lozano G (August 1990). "Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene". Science. 249 (4972): 1049–1051. Bibcode:1990Sci...249.1049R. doi:10.1126/science.2144364. PMC 2935288. PMID 2144364.
- ^ Maltzman W, Czyzyk L (September 1984). "UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells". Molecular and Cellular Biology. 4 (9): 1689–94. doi:10.1128/mcb.4.9.1689. PMC 368974. PMID 6092932.
- ^ Kastan MB, Kuerbitz SJ (December 1993). "Control of G1 arrest after DNA damage". Environmental Health Perspectives. 101 (Suppl 5): 55–8. doi:10.2307/3431842. JSTOR 3431842. PMC 1519427. PMID 8013425.
- ^ Koshland DE (December 1993). "Molecule of the year". Science. 262 (5142): 1953. Bibcode:1993Sci...262.1953K. doi:10.1126/science.8266084. PMID 8266084.
- ^ Venot C, Maratrat M, Dureuil C, et al. (August 1998). "The requirement for the p53 proline-rich functional domain for mediation of apoptosis is correlated with specific PIG3 gene transactivation and with transcriptional repression". The EMBO Journal. 17 (16): 4668–79. doi:10.1093/emboj/17.16.4668. PMC 1170796. PMID 9707426.
- ^ a b Larsen S, Yokochi T, Isogai E, et al. (February 2010). "LMO3 interacts with p53 and inhibits its transcriptional activity". Biochemical and Biophysical Research Communications. 392 (3): 252–7. doi:10.1016/j.bbrc.2009.12.010. PMID 19995558.
- ^ Harms KL, Chen X (March 2005). "The C terminus of p53 family proteins is a cell fate determinant". Molecular and Cellular Biology. 25 (5): 2014–30. doi:10.1128/MCB.25.5.2014-2030.2005. PMC 549381. PMID 15713654.
- ^ Bell S, Klein C, Müller L, et al. (October 2002). "p53 contains large unstructured regions in its native state". Journal of Molecular Biology. 322 (5): 917–27. doi:10.1016/S0022-2836(02)00848-3. PMID 12367518.
- ^ Ziemer MA, Mason A, Carlson DM (September 1982). "Cell-free translations of proline-rich protein mRNAs". The Journal of Biological Chemistry. 257 (18): 11176–80. doi:10.1016/S0021-9258(18)33948-6. PMID 7107651.
- ^ Zhu J, Zhang S, Jiang J, et al. (December 2000). "Definition of the p53 functional domains necessary for inducing apoptosis". The Journal of Biological Chemistry. 275 (51): 39927–34. doi:10.1074/jbc.M005676200. PMID 10982799.
- ^ a b Han JM, Park BJ, Park SG, et al. (August 2008). "AIMP2/p38, the scaffold for the multi-tRNA synthetase complex, responds to genotoxic stresses via p53". Proceedings of the National Academy of Sciences of the United States of America. 105 (32): 11206–11. Bibcode:2008PNAS..10511206H. doi:10.1073/pnas.0800297105. PMC 2516205. PMID 18695251.
- ^ a b Kojic S, Medeot E, Guccione E, et al. (May 2004). "The Ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle". Journal of Molecular Biology. 339 (2): 313–25. doi:10.1016/j.jmb.2004.03.071. PMID 15136035.
- ^ a b Gueven N, Becherel OJ, Kijas AW, et al. (May 2004). "Aprataxin, a novel protein that protects against genotoxic stress". Human Molecular Genetics. 13 (10): 1081–93. doi:10.1093/hmg/ddh122. PMID 15044383.
- ^ a b Fabbro M, Savage K, Hobson K, et al. (July 2004). "BRCA1-BARD1 complexes are required for p53Ser-15 phosphorylation and a G1/S arrest following ionizing radiation-induced DNA damage". The Journal of Biological Chemistry. 279 (30): 31251–8. doi:10.1074/jbc.M405372200. PMID 15159397.
- ^ a b c Kim ST, Lim DS, Canman CE, et al. (December 1999). "Substrate specificities and identification of putative substrates of ATM kinase family members". The Journal of Biological Chemistry. 274 (53): 37538–43. doi:10.1074/jbc.274.53.37538. PMID 10608806.
- ^ Kang J, Ferguson D, Song H, et al. (January 2005). "Functional interaction of H2AX, NBS1, and p53 in ATM-dependent DNA damage responses and tumor suppression". Molecular and Cellular Biology. 25 (2): 661–70. doi:10.1128/MCB.25.2.661-670.2005. PMC 543410. PMID 15632067.
- ^ Khanna KK, Keating KE, Kozlov S, et al. (December 1998). "ATM associates with and phosphorylates p53: mapping the region of interaction". Nature Genetics. 20 (4): 398–400. doi:10.1038/3882. PMID 9843217. S2CID 23994762.
- ^ Westphal CH, Schmaltz C, Rowan S, et al. (May 1997). "Genetic interactions between atm and p53 influence cellular proliferation and irradiation-induced cell cycle checkpoints". Cancer Research. 57 (9): 1664–7. PMID 9135004.
- ^ Stelzl U, Worm U, Lalowski M, et al. (September 2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. hdl:11858/00-001M-0000-0010-8592-0. PMID 16169070.
- ^ Yan C, Wang H, Boyd DD (March 2002). "ATF3 represses 72-kDa type IV collagenase (MMP-2) expression by antagonizing p53-dependent trans-activation of the collagenase promoter". The Journal of Biological Chemistry. 277 (13): 10804–12. doi:10.1074/jbc.M112069200. PMID 11792711.
- ^ Chen SS, Chang PC, Cheng YW, et al. (September 2002). "Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function". The EMBO Journal. 21 (17): 4491–9. doi:10.1093/emboj/cdf409. PMC 126178. PMID 12198151.
- ^ Leu JI, Dumont P, Hafey M, et al. (May 2004). "Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex". Nature Cell Biology. 6 (5): 443–50. doi:10.1038/ncb1123. PMID 15077116. S2CID 43063712.
- ^ a b c d e f Dong Y, Hakimi MA, Chen X, et al. (November 2003). "Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair". Molecular Cell. 12 (5): 1087–99. doi:10.1016/S1097-2765(03)00424-6. PMID 14636569.
- ^ a b c Sengupta S, Robles AI, Linke SP, et al. (September 2004). "Functional interaction between BLM helicase and 53BP1 in a Chk1-mediated pathway during S-phase arrest". The Journal of Cell Biology. 166 (6): 801–13. doi:10.1083/jcb.200405128. PMC 2172115. PMID 15364958.
- ^ Wang XW, Tseng A, Ellis NA, et al. (August 2001). "Functional interaction of p53 and BLM DNA helicase in apoptosis". The Journal of Biological Chemistry. 276 (35): 32948–55. doi:10.1074/jbc.M103298200. PMID 11399766.
- ^ Garkavtsev IV, Kley N, Grigorian IA, et al. (December 2001). "The Bloom syndrome protein interacts and cooperates with p53 in regulation of transcription and cell growth control". Oncogene. 20 (57): 8276–80. doi:10.1038/sj.onc.1205120. PMID 11781842. S2CID 13084911.
- ^ a b Yang Q, Zhang R, Wang XW, et al. (August 2002). "The processing of Holliday junctions by BLM and WRN helicases is regulated by p53". The Journal of Biological Chemistry. 277 (35): 31980–7. doi:10.1074/jbc.M204111200. hdl:10026.1/10341. PMID 12080066.
- ^ Abramovitch S, Werner H (2003). "Functional and physical interactions between BRCA1 and p53 in transcriptional regulation of the IGF-IR gene". Hormone and Metabolic Research. 35 (11–12): 758–62. doi:10.1055/s-2004-814154. PMID 14710355. S2CID 20898175.
- ^ Ouchi T, Monteiro AN, August A, et al. (March 1998). "BRCA1 regulates p53-dependent gene expression". Proceedings of the National Academy of Sciences of the United States of America. 95 (5): 2302–6. Bibcode:1998PNAS...95.2302O. doi:10.1073/pnas.95.5.2302. PMC 19327. PMID 9482880.
- ^ Chai YL, Cui J, Shao N, et al. (January 1999). "The second BRCT domain of BRCA1 proteins interacts with p53 and stimulates transcription from the p21WAF1/CIP1 promoter". Oncogene. 18 (1): 263–8. doi:10.1038/sj.onc.1202323. PMID 9926942. S2CID 7462625.
- ^ Zhang H, Somasundaram K, Peng Y, et al. (April 1998). "BRCA1 physically associates with p53 and stimulates its transcriptional activity". Oncogene. 16 (13): 1713–21. doi:10.1038/sj.onc.1201932. PMID 9582019. S2CID 24616900.
- ^ Marmorstein LY, Ouchi T, Aaronson SA (November 1998). "The BRCA2 gene product functionally interacts with p53 and RAD51". Proceedings of the National Academy of Sciences of the United States of America. 95 (23): 13869–74. Bibcode:1998PNAS...9513869M. doi:10.1073/pnas.95.23.13869. PMC 24938. PMID 9811893.
- ^ Uramoto H, Izumi H, Nagatani G, et al. (April 2003). "Physical interaction of tumour suppressor p53/p73 with CCAAT-binding transcription factor 2 (CTF2) and differential regulation of human high-mobility group 1 (HMG1) gene expression". The Biochemical Journal. 371 (Pt 2): 301–10. doi:10.1042/BJ20021646. PMC 1223307. PMID 12534345.
- ^ a b Li L, Ljungman M, Dixon JE (January 2000). "The human Cdc14 phosphatases interact with and dephosphorylate the tumor suppressor protein p53". The Journal of Biological Chemistry. 275 (4): 2410–4. doi:10.1074/jbc.275.4.2410. PMID 10644693.
- ^ Luciani MG, Hutchins JR, Zheleva D, et al. (July 2000). "The C-terminal regulatory domain of p53 contains a functional docking site for cyclin A". Journal of Molecular Biology. 300 (3): 503–18. doi:10.1006/jmbi.2000.3830. PMID 10884347.
- ^ Ababneh M, Götz C, Montenarh M (May 2001). "Downregulation of the cdc2/cyclin B protein kinase activity by binding of p53 to p34(cdc2)". Biochemical and Biophysical Research Communications. 283 (2): 507–12. doi:10.1006/bbrc.2001.4792. PMID 11327730.
- ^ Abedini MR, Muller EJ, Brun J, et al. (June 2008). "Cisplatin induces p53-dependent FLICE-like inhibitory protein ubiquitination in ovarian cancer cells". Cancer Research. 68 (12): 4511–7. doi:10.1158/0008-5472.CAN-08-0673. PMID 18559494.
- ^ a b Goudelock DM, Jiang K, Pereira E, et al. (August 2003). "Regulatory interactions between the checkpoint kinase Chk1 and the proteins of the DNA-dependent protein kinase complex". The Journal of Biological Chemistry. 278 (32): 29940–7. doi:10.1074/jbc.M301765200. PMID 12756247.
- ^ Tian H, Faje AT, Lee SL, et al. (2002). "Radiation-induced phosphorylation of Chk1 at S345 is associated with p53-dependent cell cycle arrest pathways". Neoplasia. 4 (2): 171–80. doi:10.1038/sj.neo.7900219. PMC 1550321. PMID 11896572.
- ^ Zhao L, Samuels T, Winckler S, et al. (January 2003). "Cyclin G1 has growth inhibitory activity linked to the ARF-Mdm2-p53 and pRb tumor suppressor pathways". Molecular Cancer Research. 1 (3): 195–206. PMID 12556559.
- ^ a b Ito A, Kawaguchi Y, Lai CH, et al. (November 2002). "MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation". The EMBO Journal. 21 (22): 6236–45. doi:10.1093/emboj/cdf616. PMC 137207. PMID 12426395.
- ^ a b Livengood JA, Scoggin KE, Van Orden K, et al. (March 2002). "p53 Transcriptional activity is mediated through the SRC1-interacting domain of CBP/p300". The Journal of Biological Chemistry. 277 (11): 9054–61. doi:10.1074/jbc.M108870200. PMID 11782467.
- ^ a b Giebler HA, Lemasson I, Nyborg JK (July 2000). "p53 recruitment of CREB binding protein mediated through phosphorylated CREB: a novel pathway of tumor suppressor regulation". Molecular and Cellular Biology. 20 (13): 4849–58. doi:10.1128/MCB.20.13.4849-4858.2000. PMC 85936. PMID 10848610.
- ^ a b Schneider E, Montenarh M, Wagner P (November 1998). "Regulation of CAK kinase activity by p53". Oncogene. 17 (21): 2733–41. doi:10.1038/sj.onc.1202504. PMID 9840937. S2CID 6281777.
- ^ a b Ko LJ, Shieh SY, Chen X, et al. (December 1997). "p53 is phosphorylated by CDK7-cyclin H in a p36MAT1-dependent manner". Molecular and Cellular Biology. 17 (12): 7220–9. doi:10.1128/mcb.17.12.7220. PMC 232579. PMID 9372954.
- ^ Yavuzer U, Smith GC, Bliss T, et al. (July 1998). "DNA end-independent activation of DNA-PK mediated via association with the DNA-binding protein C1D". Genes & Development. 12 (14): 2188–99. doi:10.1101/gad.12.14.2188. PMC 317006. PMID 9679063.
- ^ a b Rizos H, Diefenbach E, Badhwar P, et al. (February 2003). "Association of p14ARF with the p120E4F transcriptional repressor enhances cell cycle inhibition". The Journal of Biological Chemistry. 278 (7): 4981–9. doi:10.1074/jbc.M210978200. PMID 12446718.
- ^ Sandy P, Gostissa M, Fogal V, et al. (January 2000). "p53 is involved in the p120E4F-mediated growth arrest". Oncogene. 19 (2): 188–99. doi:10.1038/sj.onc.1203250. PMID 10644996.
- ^ a b c Gallagher WM, Argentini M, Sierra V, et al. (June 1999). "MBP1: a novel mutant p53-specific protein partner with oncogenic properties". Oncogene. 18 (24): 3608–16. doi:10.1038/sj.onc.1202937. PMID 10380882.
- ^ Cuddihy AR, Wong AH, Tam NW, et al. (April 1999). "The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro". Oncogene. 18 (17): 2690–702. doi:10.1038/sj.onc.1202620. PMID 10348343. S2CID 22467088.
- ^ Shinobu N, Maeda T, Aso T, et al. (June 1999). "Physical interaction and functional antagonism between the RNA polymerase II elongation factor ELL and p53". The Journal of Biological Chemistry. 274 (24): 17003–10. doi:10.1074/jbc.274.24.17003. PMID 10358050.
- ^ Grossman SR, Perez M, Kung AL, et al. (October 1998). "p300/MDM2 complexes participate in MDM2-mediated p53 degradation". Molecular Cell. 2 (4): 405–15. doi:10.1016/S1097-2765(00)80140-9. PMID 9809062.
- ^ An W, Kim J, Roeder RG (June 2004). "Ordered cooperative functions of PRMT1, p300, and CARM1 in transcriptional activation by p53". Cell. 117 (6): 735–48. doi:10.1016/j.cell.2004.05.009. PMID 15186775.
- ^ Pastorcic M, Das HK (November 2000). "Regulation of transcription of the human presenilin-1 gene by ets transcription factors and the p53 protooncogene". The Journal of Biological Chemistry. 275 (45): 34938–45. doi:10.1074/jbc.M005411200. PMID 10942770.
- ^ a b Wang XW, Yeh H, Schaeffer L, et al. (June 1995). "p53 modulation of TFIIH-associated nucleotide excision repair activity". Nature Genetics. 10 (2): 188–95. doi:10.1038/ng0695-188. hdl:1765/54884. PMID 7663514. S2CID 38325851.
- ^ Yu A, Fan HY, Liao D, et al. (May 2000). "Activation of p53 or loss of the Cockayne syndrome group B repair protein causes metaphase fragility of human U1, U2, and 5S genes". Molecular Cell. 5 (5): 801–10. doi:10.1016/S1097-2765(00)80320-2. PMID 10882116.
- ^ Tsai RY, McKay RD (December 2002). "A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells". Genes & Development. 16 (23): 2991–3003. doi:10.1101/gad.55671. PMC 187487. PMID 12464630.
- ^ Peng YC, Kuo F, Breiding DE, et al. (September 2001). "AMF1 (GPS2) modulates p53 transactivation". Molecular and Cellular Biology. 21 (17): 5913–24. doi:10.1128/MCB.21.17.5913-5924.2001. PMC 87310. PMID 11486030.
- ^ Watcharasit P, Bijur GN, Zmijewski JW, et al. (June 2002). "Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage". Proceedings of the National Academy of Sciences of the United States of America. 99 (12): 7951–5. Bibcode:2002PNAS...99.7951W. doi:10.1073/pnas.122062299. PMC 123001. PMID 12048243.
- ^ a b Akakura S, Yoshida M, Yoneda Y, et al. (May 2001). "A role for Hsc70 in regulating nucleocytoplasmic transport of a temperature-sensitive p53 (p53Val-135)". The Journal of Biological Chemistry. 276 (18): 14649–57. doi:10.1074/jbc.M100200200. PMID 11297531.
- ^ Wang C, Chen J (January 2003). "Phosphorylation and hsp90 binding mediate heat shock stabilization of p53". The Journal of Biological Chemistry. 278 (3): 2066–71. doi:10.1074/jbc.M206697200. PMID 12427754.
- ^ Peng Y, Chen L, Li C, et al. (November 2001). "Inhibition of MDM2 by hsp90 contributes to mutant p53 stabilization". The Journal of Biological Chemistry. 276 (44): 40583–90. doi:10.1074/jbc.M102817200. PMID 11507088.
- ^ Chen D, Li M, Luo J, et al. (April 2003). "Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function". The Journal of Biological Chemistry. 278 (16): 13595–8. doi:10.1074/jbc.C200694200. PMID 12606552.
- ^ Ravi R, Mookerjee B, Bhujwalla ZM, et al. (January 2000). "Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha". Genes & Development. 14 (1): 34–44. doi:10.1101/gad.14.1.34. PMC 316350. PMID 10640274.
- ^ Hansson LO, Friedler A, Freund S, et al. (August 2002). "Two sequence motifs from HIF-1alpha bind to the DNA-binding site of p53". Proceedings of the National Academy of Sciences of the United States of America. 99 (16): 10305–9. Bibcode:2002PNAS...9910305H. doi:10.1073/pnas.122347199. PMC 124909. PMID 12124396.
- ^ An WG, Kanekal M, Simon MC, et al. (March 1998). "Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha". Nature. 392 (6674): 405–8. Bibcode:1998Natur.392..405A. doi:10.1038/32925. PMID 9537326. S2CID 4423081.
- ^ Kondo S, Lu Y, Debbas M, et al. (April 2003). "Characterization of cells and gene-targeted mice deficient for the p53-binding kinase homeodomain-interacting protein kinase 1 (HIPK1)". Proceedings of the National Academy of Sciences of the United States of America. 100 (9): 5431–6. Bibcode:2003PNAS..100.5431K. doi:10.1073/pnas.0530308100. PMC 154362. PMID 12702766.
- ^ Hofmann TG, Möller A, Sirma H, et al. (January 2002). "Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2". Nature Cell Biology. 4 (1): 1–10. doi:10.1038/ncb715. PMID 11740489. S2CID 37789883.
- ^ Kim EJ, Park JS, Um SJ (August 2002). "Identification and characterization of HIPK2 interacting with p73 and modulating functions of the p53 family in vivo". The Journal of Biological Chemistry. 277 (35): 32020–8. doi:10.1074/jbc.M200153200. PMID 11925430.
- ^ Imamura T, Izumi H, Nagatani G, et al. (March 2001). "Interaction with p53 enhances binding of cisplatin-modified DNA by high mobility group 1 protein". The Journal of Biological Chemistry. 276 (10): 7534–40. doi:10.1074/jbc.M008143200. PMID 11106654.
- ^ Dintilhac A, Bernués J (March 2002). "HMGB1 interacts with many apparently unrelated proteins by recognizing short amino acid sequences". The Journal of Biological Chemistry. 277 (9): 7021–8. doi:10.1074/jbc.M108417200. hdl:10261/112516. PMID 11748221.
- ^ Wadhwa R, Yaguchi T, Hasan MK, et al. (April 2002). "Hsp70 family member, mot-2/mthsp70/GRP75, binds to the cytoplasmic sequestration domain of the p53 protein". Experimental Cell Research. 274 (2): 246–53. doi:10.1006/excr.2002.5468. PMID 11900485.
- ^ Steffan JS, Kazantsev A, Spasic-Boskovic O, et al. (June 2000). "The Huntington's disease protein interacts with p53 and CREB-binding protein and represses transcription". Proceedings of the National Academy of Sciences of the United States of America. 97 (12): 6763–8. Bibcode:2000PNAS...97.6763S. doi:10.1073/pnas.100110097. PMC 18731. PMID 10823891.
- ^ Leung KM, Po LS, Tsang FC, et al. (September 2002). "The candidate tumor suppressor ING1b can stabilize p53 by disrupting the regulation of p53 by MDM2". Cancer Research. 62 (17): 4890–3. PMID 12208736.
- ^ Garkavtsev I, Grigorian IA, Ossovskaya VS, et al. (January 1998). "The candidate tumour suppressor p33ING1 cooperates with p53 in cell growth control". Nature. 391 (6664): 295–8. Bibcode:1998Natur.391..295G. doi:10.1038/34675. PMID 9440695. S2CID 4429461.
- ^ a b Shiseki M, Nagashima M, Pedeux RM, et al. (May 2003). "p29ING4 and p28ING5 bind to p53 and p300, and enhance p53 activity". Cancer Research. 63 (10): 2373–8. PMID 12750254.
- ^ Tsai KW, Tseng HC, Lin WC (October 2008). "Two wobble-splicing events affect ING4 protein subnuclear localization and degradation". Experimental Cell Research. 314 (17): 3130–41. doi:10.1016/j.yexcr.2008.08.002. PMID 18775696.
- ^ Chang NS (March 2002). "The non-ankyrin C terminus of Ikappa Balpha physically interacts with p53 in vivo and dissociates in response to apoptotic stress, hypoxia, DNA damage, and transforming growth factor-beta 1-mediated growth suppression". The Journal of Biological Chemistry. 277 (12): 10323–31. doi:10.1074/jbc.M106607200. PMID 11799106.
- ^ a b Kurki S, Latonen L, Laiho M (October 2003). "Cellular stress and DNA damage invoke temporally distinct Mdm2, p53 and PML complexes and damage-specific nuclear relocalization". Journal of Cell Science. 116 (Pt 19): 3917–25. doi:10.1242/jcs.00714. PMID 12915590.
- ^ a b Freeman DJ, Li AG, Wei G, et al. (February 2003). "PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms". Cancer Cell. 3 (2): 117–30. doi:10.1016/S1535-6108(03)00021-7. PMID 12620407.
- ^ a b Zhang Y, Xiong Y, Yarbrough WG (March 1998). "ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways". Cell. 92 (6): 725–34. doi:10.1016/S0092-8674(00)81401-4. PMID 9529249.
- ^ Badciong JC, Haas AL (December 2002). "MdmX is a RING finger ubiquitin ligase capable of synergistically enhancing Mdm2 ubiquitination". The Journal of Biological Chemistry. 277 (51): 49668–75. doi:10.1074/jbc.M208593200. PMID 12393902.
- ^ Shvarts A, Bazuine M, Dekker P, et al. (July 1997). "Isolation and identification of the human homolog of a new p53-binding protein, Mdmx" (PDF). Genomics. 43 (1): 34–42. doi:10.1006/geno.1997.4775. hdl:2066/142231. PMID 9226370. S2CID 11794685.
- ^ Frade R, Balbo M, Barel M (December 2000). "RB18A, whose gene is localized on chromosome 17q12-q21.1, regulates in vivo p53 transactivating activity". Cancer Research. 60 (23): 6585–9. PMID 11118038.
- ^ Drané P, Barel M, Balbo M, et al. (December 1997). "Identification of RB18A, a 205 kDa new p53 regulatory protein which shares antigenic and functional properties with p53". Oncogene. 15 (25): 3013–24. doi:10.1038/sj.onc.1201492. PMID 9444950.
- ^ Hu MC, Qiu WR, Wang YP (November 1997). "JNK1, JNK2 and JNK3 are p53 N-terminal serine 34 kinases". Oncogene. 15 (19): 2277–87. doi:10.1038/sj.onc.1201401. PMID 9393873.
- ^ Lin Y, Khokhlatchev A, Figeys D, et al. (December 2002). "Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis". The Journal of Biological Chemistry. 277 (50): 47991–8001. doi:10.1074/jbc.M202630200. PMID 12384512.
- ^ Taniura H, Matsumoto K, Yoshikawa K (June 1999). "Physical and functional interactions of neuronal growth suppressor necdin with p53". The Journal of Biological Chemistry. 274 (23): 16242–8. doi:10.1074/jbc.274.23.16242. PMID 10347180.
- ^ Daniely Y, Dimitrova DD, Borowiec JA (August 2002). "Stress-dependent nucleolin mobilization mediated by p53-nucleolin complex formation". Molecular and Cellular Biology. 22 (16): 6014–22. doi:10.1128/MCB.22.16.6014-6022.2002. PMC 133981. PMID 12138209.
- ^ Colaluca IN, Tosoni D, Nuciforo P, et al. (January 2008). "NUMB controls p53 tumour suppressor activity". Nature. 451 (7174): 76–80. Bibcode:2008Natur.451...76C. doi:10.1038/nature06412. PMID 18172499. S2CID 4431258.
- ^ a b c Choy MK, Movassagh M, Siggens L, et al. (June 2010). "High-throughput sequencing identifies STAT3 as the DNA-associated factor for p53-NF-kappaB-complex-dependent gene expression in human heart failure". Genome Medicine. 2 (6): 37. doi:10.1186/gm158. PMC 2905097. PMID 20546595.
- ^ a b Zhang Y, Wolf GW, Bhat K, et al. (December 2003). "Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway". Molecular and Cellular Biology. 23 (23): 8902–12. doi:10.1128/MCB.23.23.8902-8912.2003. PMC 262682. PMID 14612427.
- ^ Nikolaev AY, Li M, Puskas N, et al. (January 2003). "Parc: a cytoplasmic anchor for p53". Cell. 112 (1): 29–40. doi:10.1016/S0092-8674(02)01255-2. PMID 12526791.
- ^ Malanga M, Pleschke JM, Kleczkowska HE, et al. (May 1998). "Poly(ADP-ribose) binds to specific domains of p53 and alters its DNA binding functions". The Journal of Biological Chemistry. 273 (19): 11839–43. doi:10.1074/jbc.273.19.11839. PMID 9565608.
- ^ Kahyo T, Nishida T, Yasuda H (September 2001). "Involvement of PIAS1 in the sumoylation of tumor suppressor p53". Molecular Cell. 8 (3): 713–8. doi:10.1016/S1097-2765(01)00349-5. PMID 11583632.
- ^ Wulf GM, Liou YC, Ryo A, et al. (December 2002). "Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage". The Journal of Biological Chemistry. 277 (50): 47976–9. doi:10.1074/jbc.C200538200. PMID 12388558.
- ^ Zacchi P, Gostissa M, Uchida T, et al. (October 2002). "The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults". Nature. 419 (6909): 853–7. Bibcode:2002Natur.419..853Z. doi:10.1038/nature01120. PMID 12397362. S2CID 4311658.
- ^ Huang SM, Schönthal AH, Stallcup MR (April 2001). "Enhancement of p53-dependent gene activation by the transcriptional coactivator Zac1". Oncogene. 20 (17): 2134–43. doi:10.1038/sj.onc.1204298. PMID 11360197. S2CID 21331603.
- ^ Xie S, Wu H, Wang Q, et al. (November 2001). "Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway". The Journal of Biological Chemistry. 276 (46): 43305–12. doi:10.1074/jbc.M106050200. PMID 11551930.
- ^ Bahassi EM, Conn CW, Myer DL, et al. (September 2002). "Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways". Oncogene. 21 (43): 6633–40. doi:10.1038/sj.onc.1205850. PMID 12242661. S2CID 24106070.
- ^ Simons A, Melamed-Bessudo C, Wolkowicz R, et al. (January 1997). "PACT: cloning and characterization of a cellular p53 binding protein that interacts with Rb". Oncogene. 14 (2): 145–55. doi:10.1038/sj.onc.1200825. PMID 9010216.
- ^ Fusaro G, Dasgupta P, Rastogi S, et al. (November 2003). "Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling". The Journal of Biological Chemistry. 278 (48): 47853–61. doi:10.1074/jbc.M305171200. PMID 14500729.
- ^ Fogal V, Gostissa M, Sandy P, et al. (November 2000). "Regulation of p53 activity in nuclear bodies by a specific PML isoform". The EMBO Journal. 19 (22): 6185–95. doi:10.1093/emboj/19.22.6185. PMC 305840. PMID 11080164.
- ^ Guo A, Salomoni P, Luo J, et al. (October 2000). "The function of PML in p53-dependent apoptosis". Nature Cell Biology. 2 (10): 730–6. doi:10.1038/35036365. PMID 11025664. S2CID 13480833.
- ^ a b Zhang Z, Zhang R (March 2008). "Proteasome activator PA28 gamma regulates p53 by enhancing its MDM2-mediated degradation". The EMBO Journal. 27 (6): 852–64. doi:10.1038/emboj.2008.25. PMC 2265109. PMID 18309296.
- ^ Lim ST, Chen XL, Lim Y, et al. (January 2008). "Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation". Molecular Cell. 29 (1): 9–22. doi:10.1016/j.molcel.2007.11.031. PMC 2234035. PMID 18206965.
- ^ Bernal JA, Luna R, Espina A, et al. (October 2002). "Human securin interacts with p53 and modulates p53-mediated transcriptional activity and apoptosis". Nature Genetics. 32 (2): 306–11. doi:10.1038/ng997. PMID 12355087. S2CID 1770399.
- ^ Stürzbecher HW, Donzelmann B, Henning W, et al. (April 1996). "p53 is linked directly to homologous recombination processes via RAD51/RecA protein interaction". The EMBO Journal. 15 (8): 1992–2002. doi:10.1002/j.1460-2075.1996.tb00550.x. PMC 450118. PMID 8617246.
- ^ Buchhop S, Gibson MK, Wang XW, et al. (October 1997). "Interaction of p53 with the human Rad51 protein". Nucleic Acids Research. 25 (19): 3868–74. doi:10.1093/nar/25.19.3868. PMC 146972. PMID 9380510.
- ^ Leng RP, Lin Y, Ma W, et al. (March 2003). "Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation". Cell. 112 (6): 779–91. doi:10.1016/S0092-8674(03)00193-4. PMID 12654245.
- ^ Sheng Y, Laister RC, Lemak A, et al. (December 2008). "Molecular basis of Pirh2-mediated p53 ubiquitylation". Nature Structural & Molecular Biology. 15 (12): 1334–42. doi:10.1038/nsmb.1521. PMC 4075976. PMID 19043414.
- ^ Romanova LY, Willers H, Blagosklonny MV, et al. (December 2004). "The interaction of p53 with replication protein A mediates suppression of homologous recombination". Oncogene. 23 (56): 9025–33. doi:10.1038/sj.onc.1207982. PMID 15489903. S2CID 23482723.
- ^ Riva F, Zuco V, Vink AA, et al. (December 2001). "UV-induced DNA incision and proliferating cell nuclear antigen recruitment to repair sites occur independently of p53-replication protein A interaction in p53 wild type and mutant ovarian carcinoma cells". Carcinogenesis. 22 (12): 1971–8. doi:10.1093/carcin/22.12.1971. PMID 11751427.
- ^ Lin J, Yang Q, Yan Z, et al. (August 2004). "Inhibiting S100B restores p53 levels in primary malignant melanoma cancer cells". The Journal of Biological Chemistry. 279 (32): 34071–7. doi:10.1074/jbc.M405419200. PMID 15178678.
- ^ a b Minty A, Dumont X, Kaghad M, et al. (November 2000). "Covalent modification of p73alpha by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif". The Journal of Biological Chemistry. 275 (46): 36316–23. doi:10.1074/jbc.M004293200. PMID 10961991.
- ^ a b Ivanchuk SM, Mondal S, Rutka JT (June 2008). "p14ARF interacts with DAXX: effects on HDM2 and p53". Cell Cycle. 7 (12): 1836–50. doi:10.4161/cc.7.12.6025. PMID 18583933.
- ^ a b Lee D, Kim JW, Seo T, et al. (June 2002). "SWI/SNF complex interacts with tumor suppressor p53 and is necessary for the activation of p53-mediated transcription". The Journal of Biological Chemistry. 277 (25): 22330–7. doi:10.1074/jbc.M111987200. PMID 11950834.
- ^ Young PJ, Day PM, Zhou J, et al. (January 2002). "A direct interaction between the survival motor neuron protein and p53 and its relationship to spinal muscular atrophy". The Journal of Biological Chemistry. 277 (4): 2852–9. doi:10.1074/jbc.M108769200. PMID 11704667.
- ^ Seto E, Usheva A, Zambetti GP, et al. (December 1992). "Wild-type p53 binds to the TATA-binding protein and represses transcription". Proceedings of the National Academy of Sciences of the United States of America. 89 (24): 12028–32. Bibcode:1992PNAS...8912028S. doi:10.1073/pnas.89.24.12028. PMC 50691. PMID 1465435.
- ^ Cvekl A, Kashanchi F, Brady JN, et al. (June 1999). "Pax-6 interactions with TATA-box-binding protein and retinoblastoma protein". Investigative Ophthalmology & Visual Science. 40 (7): 1343–50. PMID 10359315.
- ^ McPherson LA, Loktev AV, Weigel RJ (November 2002). "Tumor suppressor activity of AP2alpha mediated through a direct interaction with p53". The Journal of Biological Chemistry. 277 (47): 45028–33. doi:10.1074/jbc.M208924200. PMID 12226108.
- ^ Sørensen TS, Girling R, Lee CW, et al. (October 1996). "Functional interaction between DP-1 and p53". Molecular and Cellular Biology. 16 (10): 5888–95. doi:10.1128/mcb.16.10.5888. PMC 231590. PMID 8816502.
- ^ Green DR, Chipuk JE (July 2006). "p53 and metabolism: Inside the TIGAR". Cell. 126 (1): 30–2. doi:10.1016/j.cell.2006.06.032. PMID 16839873.
- ^ Gobert C, Skladanowski A, Larsen AK (August 1999). "The interaction between p53 and DNA topoisomerase I is regulated differently in cells with wild-type and mutant p53". Proceedings of the National Academy of Sciences of the United States of America. 96 (18): 10355–60. Bibcode:1999PNAS...9610355G. doi:10.1073/pnas.96.18.10355. PMC 17892. PMID 10468612.
- ^ Mao Y, Mehl IR, Muller MT (February 2002). "Subnuclear distribution of topoisomerase I is linked to ongoing transcription and p53 status". Proceedings of the National Academy of Sciences of the United States of America. 99 (3): 1235–40. Bibcode:2002PNAS...99.1235M. doi:10.1073/pnas.022631899. PMC 122173. PMID 11805286.
- ^ a b Cowell IG, Okorokov AL, Cutts SA, et al. (February 2000). "Human topoisomerase IIalpha and IIbeta interact with the C-terminal region of p53". Experimental Cell Research. 255 (1): 86–94. doi:10.1006/excr.1999.4772. PMID 10666337.
- ^ Derbyshire DJ, Basu BP, Serpell LC, et al. (July 2002). "Crystal structure of human 53BP1 BRCT domains bound to p53 tumour suppressor". The EMBO Journal. 21 (14): 3863–72. doi:10.1093/emboj/cdf383. PMC 126127. PMID 12110597.
- ^ Ekblad CM, Friedler A, Veprintsev D, et al. (March 2004). "Comparison of BRCT domains of BRCA1 and 53BP1: a biophysical analysis". Protein Science. 13 (3): 617–25. doi:10.1110/ps.03461404. PMC 2286730. PMID 14978302.
- ^ Lo KW, Kan HM, Chan LN, et al. (March 2005). "The 8-kDa dynein light chain binds to p53-binding protein 1 and mediates DNA damage-induced p53 nuclear accumulation". The Journal of Biological Chemistry. 280 (9): 8172–9. doi:10.1074/jbc.M411408200. PMID 15611139.
- ^ Joo WS, Jeffrey PD, Cantor SB, et al. (March 2002). "Structure of the 53BP1 BRCT region bound to p53 and its comparison to the Brca1 BRCT structure". Genes & Development. 16 (5): 583–93. doi:10.1101/gad.959202. PMC 155350. PMID 11877378.
- ^ Derbyshire DJ, Basu BP, Date T, et al. (October 2002). "Purification, crystallization and preliminary X-ray analysis of the BRCT domains of human 53BP1 bound to the p53 tumour suppressor". Acta Crystallographica D. 58 (Pt 10 Pt 2): 1826–9. Bibcode:2002AcCrD..58.1826D. doi:10.1107/S0907444902010910. PMID 12351827.
- ^ a b Iwabuchi K, Bartel PL, Li B, et al. (June 1994). "Two cellular proteins that bind to wild-type but not mutant p53". Proceedings of the National Academy of Sciences of the United States of America. 91 (13): 6098–102. Bibcode:1994PNAS...91.6098I. doi:10.1073/pnas.91.13.6098. PMC 44145. PMID 8016121.
- ^ Naumovski L, Cleary ML (July 1996). "The p53-binding protein 53BP2 also interacts with Bc12 and impedes cell cycle progression at G2/M". Molecular and Cellular Biology. 16 (7): 3884–92. doi:10.1128/MCB.16.7.3884. PMC 231385. PMID 8668206.
- ^ Tomasini R, Samir AA, Carrier A, et al. (September 2003). "TP53INP1s and homeodomain-interacting protein kinase-2 (HIPK2) are partners in regulating p53 activity". The Journal of Biological Chemistry. 278 (39): 37722–9. doi:10.1074/jbc.M301979200. PMID 12851404.
- ^ Okamura S, Arakawa H, Tanaka T, et al. (July 2001). "p53DINP1, a p53-inducible gene, regulates p53-dependent apoptosis". Molecular Cell. 8 (1): 85–94. doi:10.1016/S1097-2765(01)00284-2. PMID 11511362.
- ^ Li L, Liao J, Ruland J, et al. (February 2001). "A TSG101/MDM2 regulatory loop modulates MDM2 degradation and MDM2/p53 feedback control". Proceedings of the National Academy of Sciences of the United States of America. 98 (4): 1619–24. Bibcode:2001PNAS...98.1619L. doi:10.1073/pnas.98.4.1619. PMC 29306. PMID 11172000.
- ^ Lyakhovich A, Shekhar MP (April 2003). "Supramolecular complex formation between Rad6 and proteins of the p53 pathway during DNA damage-induced response". Molecular and Cellular Biology. 23 (7): 2463–75. doi:10.1128/MCB.23.7.2463-2475.2003. PMC 150718. PMID 12640129.
- ^ Shen Z, Pardington-Purtymun PE, Comeaux JC, et al. (October 1996). "Associations of UBE2I with RAD52, UBL1, p53, and RAD51 proteins in a yeast two-hybrid system". Genomics. 37 (2): 183–6. doi:10.1006/geno.1996.0540. PMID 8921390.
- ^ Bernier-Villamor V, Sampson DA, Matunis MJ, et al. (February 2002). "Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1". Cell. 108 (3): 345–56. doi:10.1016/S0092-8674(02)00630-X. PMID 11853669.
- ^ Sehat B, Andersson S, Girnita L, et al. (July 2008). "Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis". Cancer Research. 68 (14): 5669–77. doi:10.1158/0008-5472.CAN-07-6364. PMID 18632619.
- ^ Song MS, Song SJ, Kim SY, et al. (July 2008). "The tumour suppressor RASSF1A promotes MDM2 self-ubiquitination by disrupting the MDM2-DAXX-HAUSP complex". The EMBO Journal. 27 (13): 1863–74. doi:10.1038/emboj.2008.115. PMC 2486425. PMID 18566590.
- ^ Yang W, Dicker DT, Chen J, et al. (March 2008). "CARPs enhance p53 turnover by degrading 14-3-3sigma and stabilizing MDM2". Cell Cycle. 7 (5): 670–82. doi:10.4161/cc.7.5.5701. PMID 18382127.
- ^ Abe Y, Oda-Sato E, Tobiume K, et al. (March 2008). "Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2". Proceedings of the National Academy of Sciences of the United States of America. 105 (12): 4838–43. Bibcode:2008PNAS..105.4838A. doi:10.1073/pnas.0712216105. PMC 2290789. PMID 18359851.
- ^ Dohmesen C, Koeppel M, Dobbelstein M (January 2008). "Specific inhibition of Mdm2-mediated neddylation by Tip60". Cell Cycle. 7 (2): 222–31. doi:10.4161/cc.7.2.5185. PMID 18264029. S2CID 8023403.
- ^ Li M, Chen D, Shiloh A, et al. (April 2002). "Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization". Nature. 416 (6881): 648–53. Bibcode:2002Natur.416..648L. doi:10.1038/nature737. PMID 11923872. S2CID 4389394.
- ^ Brosh RM, Karmakar P, Sommers JA, et al. (September 2001). "p53 Modulates the exonuclease activity of Werner syndrome protein". The Journal of Biological Chemistry. 276 (37): 35093–102. doi:10.1074/jbc.M103332200. PMID 11427532.
- ^ Chang NS, Pratt N, Heath J, et al. (February 2001). "Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity". The Journal of Biological Chemistry. 276 (5): 3361–70. doi:10.1074/jbc.M007140200. PMID 11058590.
- ^ Okamoto T, Izumi H, Imamura T, et al. (December 2000). "Direct interaction of p53 with the Y-box binding protein, YB-1: a mechanism for regulation of human gene expression". Oncogene. 19 (54): 6194–202. doi:10.1038/sj.onc.1204029. PMID 11175333. S2CID 19222684.
- ^ Kelley KD, Miller KR, Todd A, et al. (May 2010). "YPEL3, a p53-regulated gene that induces cellular senescence". Cancer Research. 70 (9): 3566–75. doi:10.1158/0008-5472.CAN-09-3219. PMC 2862112. PMID 20388804.
- ^ Waterman MJ, Stavridi ES, Waterman JL, et al. (June 1998). "ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins". Nature Genetics. 19 (2): 175–8. doi:10.1038/542. PMID 9620776. S2CID 26600934.
- ^ Liu J, Grogan L, Nau MM, et al. (April 2001). "Physical interaction between p53 and primary response gene Egr-1". International Journal of Oncology. 18 (4): 863–70. doi:10.3892/ijo.18.4.863. PMID 11251186.
- ^ Bai L, Merchant JL (July 2001). "ZBP-89 promotes growth arrest through stabilization of p53". Molecular and Cellular Biology. 21 (14): 4670–83. doi:10.1128/MCB.21.14.4670-4683.2001. PMC 87140. PMID 11416144.
- ^ Yamakuchi M, Lowenstein CJ (March 2009). "MiR-34, SIRT1 and p53: the feedback loop". Cell Cycle. 8 (5): 712–5. doi:10.4161/cc.8.5.7753. PMID 19221490.
- ^ Wang Y, Zhang J, Li J, et al. (May 2019). "CircRNA_014511 affects the radiosensitivity of bone marrow mesenchymal stem cells by binding to miR-29b-2-5p". Bosnian Journal of Basic Medical Sciences. 19 (2): 155–163. doi:10.17305/bjbms.2019.3935. PMC 6535393. PMID 30640591.
External links
[edit]- "p53 Knowledgebase". Lane Group at the Institute of Molecular and Cell Biology (IMCB), Singapore. Archived from the original on 2006-01-03. Retrieved 2008-04-06.
- GeneReviews/NCBI/NIH/UW entry on Li-Fraumeni Syndrome
- TUMOR PROTEIN p53 @ OMIM
- p53 restoration of function
- p53 @ The Atlas of Genetics and Cytogenetics in Oncology and Haematology
- TP53 Gene @ GeneCards
- p53 News provided by insciences organisation
- Goodsel DS (2002-07-01). "p53 Tumor Suppressor". Molecule of the Month. RCSB Protein Data Bank. Retrieved 2008-04-06.
- Soussi T. "p53 Web Site". Retrieved 2008-04-06.
- Living LFS A non-profit Li-Fraumeni Syndrome patient support organization
- The George Pantziarka TP53 Trust A support group from the UK for people with Li-Fraumeni Syndrome or other TP53-related disorders
- IARC TP53 Somatic Mutations database maintained at IARC, Lyon, by Magali Olivier
- PDBe-KB provides an overview of all the structure information available in the PDB for Human P53.
- scientific animation conformational changes of p53 upon binding to DNA