Dronabinol
| Part of a series on |
| Cannabis |
|---|
 |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Marinol, Syndros |
| Other names | (−)-trans-Δ9-tetrahydrocannabinol |
| License data |
|
| Dependence liability | Physical: Low Psychological: Low–moderate |
| Addiction liability | Relatively low: 9% |
| Routes of administration | By mouth |
| Drug class | Cannabinoid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 6–20% |
| Onset of action | Oral: 0.5–1 hour |
| Elimination half-life | 25–36 hours |
| Duration of action | Oral: 4–6 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
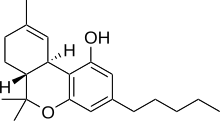
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | −152° (ethanol) |
| Boiling point | 155–157 °C (311–315 °F) 0.05mmHg,[1] 157–160°C @ 0.05mmHg[2] |
| Solubility in water | 0.0028 mg/mL (23 °C)[3] |
| |
| |
Dronabinol (INN), sold under the brand names Marinol and Syndros, is the generic name for the molecule of (−)-trans-Δ9-tetrahydrocannabinol (THC) in the pharmaceutical context. It has indications as an appetite stimulant, antiemetic, and sleep apnea reliever[4] and is approved by the U.S. Food and Drug Administration (FDA) as safe and effective for HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting.[5][6][7]
Dronabinol is the principal psychoactive constituent enantiomer form, (−)-trans-Δ9-tetrahydrocannabinol, found in Cannabis sativa L. plants,[8] but can also be synthesized in laboratory. Dronabinol does not include any other tetrahydrocannabinol (THC) isomers or any cannabidiol.
Medical uses
[edit]Low appetite and nausea
[edit]Dronabinol is used to stimulate appetite and therefore weight gain in patients with HIV/AIDS and cancer. It is also used to treat chemotherapy-induced nausea and vomiting.[9][10]
Pain
[edit]Dronabinol demonstrated analgesic efficacy in a majority of studies in chronic pain, the data in acute pain is less conclusive.[11]
Cannabis use disorder
[edit]Dronabinol may be useful in treating cannabis addiction as it has been shown to reduce cannabis withdrawal symptoms and the subjective effects of cannabis.[12]
Sleep apnea
[edit]Dronabinol demonstrates significant improvement in sleep apnea scores.[4][13][14][15] Phase 2B clinical trials were completed in 2017 for FDA approval for this indication.[16][17][18]
Adverse effects
[edit]Common side effects of dronabinol include euphoria, drowsiness, dizziness, decreased motor coordination, anxiety, paranoia, confusion, and a rapid heartbeat, among others.[19]
Overdose
[edit]In a mild overdose of dronabinol, the typical side effects are exacerbated, whereas a severe overdose presents with lethargy, slurred speech, severe ataxia, and orthostatic hypotension.[5][20]
Pharmacology
[edit]History
[edit]While dronabinol was initially approved by the United States Food and Drug Administration (FDA) on May 31, 1985,[21] it was not until May 13, 1986, the Drug Enforcement Administration (DEA), issued a Final Rule and Statement of Policy authorizing the "rescheduling of synthetic dronabinol in sesame oil and encapsulated in soft gelatin capsules from Schedule I to Schedule II" (DEA 51 FR 17476-78). This permitted medical use of Marinol, albeit with the severe restrictions associated with Schedule II status.[22] For instance, refills of Marinol prescriptions were not permitted.
On April 29, 1991, the Commission on Narcotic Drugs, in accordance with article 2, paragraphs 5 and 6, of the Convention on Psychotropic Substances of 1971, decided that Δ9-tetrahydrocannabinol (also referred to as Δ9-THC) and its stereochemical variants should be transferred from Schedule I to Schedule II of that Convention. This released Δ9-THC from many of the restrictions imposed by the convention, facilitating its marketing as medication.[23]
An article published in the April–June 1998 issue of the Journal of Psychoactive Drugs found that "Healthcare professionals have detected no indication of script-chasing or doctor-shopping among the patients for whom they have prescribed dronabinol". The authors state that Marinol has a low potential for abuse.[24][better source needed]
In 1999, in the United States, Marinol was rescheduled from Schedule II to III of the Controlled Substances Act, reflecting a finding that THC had a potential for abuse less than that of cocaine and heroin.[21] This rescheduling constituted part of the argument for a 2002 petition for removal of cannabis from Schedule I of the Controlled Substances Act, in which petitioner Jon Gettman noted, "Cannabis is a natural source of dronabinol (THC), the ingredient of Marinol, a Schedule III drug. There are no grounds to schedule cannabis in a more restrictive schedule than Marinol".[25][better source needed]
In 2003, the World Health Organization Expert Committee on Drug Dependence recommended transferring THC to Schedule IV of the convention, citing its medical uses and low abuse potential.[26] In 2019, the Committee recommended transferring Δ9-THC to Schedule I of the Single Convention on Narcotic Drugs of 1961, but its recommendations were rejected by the United Nations Commission on Narcotic Drugs.[27]
Society and culture
[edit]Brand names
[edit]Dronabinol is marketed as Marinol and Syndros,[28] a registered trademark of Solvay Pharmaceuticals. Dronabinol is also marketed, sold, and distributed by PAR Pharmaceutical Companies under the terms of a license and distribution agreement with SVC pharma LP, an affiliate of Rhodes Technologies for Marinol and Insys Pharmaceuticals for Syndros.[citation needed] Dronabinol is available as a prescription drug (under Marinol and Syndros [29]) in several countries including the United States, Germany, South Africa and Australia.[30] In Canada, Tetra Bio-Pharma filed a New Drug Submission (NDS) with Health Canada for its Dronabinol Soft Gel capsules to be marketed as REDUVO™.[31] Tetra has two other dronabinol drugs with new routes of administration which limit first-pass metabolism; an inhaled THC-based dronabinol drug and their mucoadhesive-delivery dronabinol drug Adversa®, which are both in the accelerated 505(b)(2) New Drug Application (NDA) pathway for the U.S. and Canadian markets.[32]
In the United States, Marinol is a Schedule III drug, available by prescription, considered to be non-narcotic and to have a low risk of physical or mental dependence. Efforts to get cannabis rescheduled as analogous to Marinol have not succeeded thus far, though a 2002 petition has been accepted by the DEA. As a result of the rescheduling of Marinol from Schedule II to Schedule III, refills are now permitted for this substance. Marinol's U.S. Food and Drug Administration (FDA) approval for medical use has raised much controversy[33] as to why cannabis is still illegal at the federal level.[34]
Comparisons with medical cannabis
[edit]Female cannabis plants not only contain dronabinol but at least 113 other cannabinoids,[35] including cannabidiol (CBD), thought to be the major anticonvulsant that helps people with multiple sclerosis;[36] and cannabichromene (CBC), an anti-inflammatory which may contribute to the pain-killing effect of cannabis.[37]
It takes over one hour for Marinol to reach full systemic effect,[38] compared to seconds or minutes for smoked or vaporized cannabis.[39] Mark Kleiman, director of the Drug Policy Analysis Program at UCLA's School of Public Affairs said of Marinol, "it wasn't any fun and made the user feel bad, so it could be approved without any fear that it would penetrate the recreational market, and then used as a club with which to beat back the advocates of whole cannabis as a medicine."[40]
Clinical trials comparing the use of cannabis extracts with Marinol in the treatment of cancer cachexia have demonstrated equal efficacy and well-being among subjects in the two treatment arms.[41] United States federal law currently registers dronabinol as a Schedule III controlled substance, but all other cannabinoids remain Schedule I, except various synthetic cannabinoids like nabilone and HU-308.[42][43]
See also
[edit]- Cannabinoids
- 11-Hydroxy-THC, metabolite of THC
- Anandamide, 2-Arachidonoylglycerol, endogenous cannabinoids
- Tetrahydrocannabinol
- Cannabidiol (CBD)
- Cannabinol (CBN), a metabolite of THC
- Dimethylheptylpyran
- Parahexyl
- Tetrahydrocannabinolic acid, the biosynthetic precursor for THC
- HU-210, WIN 55,212-2, JWH-133, synthetic cannabinoid agonists (neocannabinoids)
- Medical cannabis (pharmaceutical cannabinoids)
- Epidiolex (prescription form of purified cannabidiol derived from hemp used for treating some rare neurological diseases)
- Sativex (nabiximols)
- Nabilone, a novel synthetic cannabinoid
- HU-308, a highly potent synthetic cannabinoid CB2 receptor agonist
References
[edit]- ^ Gaoni Y, Mechoulam R (April 1964). "Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish". Journal of the American Chemical Society. 86 (8): 1646–47. Bibcode:1964JAChS..86.1646G. doi:10.1021/ja01062a046.
- ^ Adams R, Cain CK, McPhee WD, Wearn RB (August 1941). "Structure of Cannabidiol. XII. Isomerization to Tetrahydrocannabinols". Journal of the American Chemical Society. 63 (8): 2209–13. Bibcode:1941JAChS..63.2209A. doi:10.1021/ja01853a052.
- ^ Garrett ER, Hunt CA (July 1974). "Physiochemical properties, solubility, and protein binding of delta9-tetrahydrocannabinol". Journal of Pharmaceutical Sciences. 63 (7): 1056–64. doi:10.1002/jps.2600630705. PMID 4853640.
- ^ a b Schütz SG, Dunn A, Braley TJ, Pitt B, Shelgikar AV (June 2021). "New frontiers in pharmacologic obstructive sleep apnea treatment: A narrative review". Sleep Medicine Reviews. 57. Elsevier BV: 101473. doi:10.1016/j.smrv.2021.101473. PMID 33853035. S2CID 233242139.
Initial rodent studies showed that injections of dronabinol, a synthetic form of delta-9-tetrahydrocannabinol, in the nodose ganglia suppressed serotonin induced reflex apneas and increased upper airway dilating muscle activity during sleep. Limited studies in humans with moderate-to-severe OSA have demonstrated significant reduction in AHI with dronabinol use.
- ^ a b "Marinol (Dronabinol)" (PDF). US Food and Drug Administration. September 2004. Retrieved 14 January 2018.
- ^ "Cannabis and Cannabinoids". National Cancer Institute. 2011-10-24. Retrieved 12 January 2014.
- ^ Badowski ME (September 2017). "A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: a focus on pharmacokinetic variability and pharmacodynamics". Cancer Chemotherapy and Pharmacology. 80 (3): 441–449. doi:10.1007/s00280-017-3387-5. PMC 5573753. PMID 28780725.
- ^ "List of psychotropic substances under international control". International Narcotics Control Board. Retrieved 25 April 2018.
This international non-proprietary name refers to only one of the stereochemical variants of delta-9-tetrahydrocannabinol, namely (−)-trans-delta-9-tetrahydrocannabinol
- ^ Badowski ME, Yanful PK (2018). "Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer". Therapeutics and Clinical Risk Management. 14: 643–651. doi:10.2147/TCRM.S126849. PMC 5896684. PMID 29670357.
- ^ May MB, Glode AE (2016). "Dronabinol for chemotherapy-induced nausea and vomiting unresponsive to antiemetics". Cancer Management and Research. 8. Informa PLC: 49–55. doi:10.2147/cmar.s81425. PMC 4869612. PMID 27274310.
- ^ de Vries M, van Rijckevorsel DC, Wilder-Smith OH, van Goor H (2014). "Dronabinol and chronic pain: importance of mechanistic considerations". Expert Opinion on Pharmacotherapy. 15 (11): 1525–34. doi:10.1517/14656566.2014.918102. PMID 24819592. S2CID 31008562.
- ^ Levin FR, Kleber HD (2008). "Use of dronabinol for cannabis dependence: two case reports and review". Am J Addict. 17 (2): 161–4. doi:10.1080/10550490701861177. PMC 2733248. PMID 18393061.
- ^ Prasad B, Radulovacki MG, Carley DW (2013). "Proof of concept trial of dronabinol in obstructive sleep apnea". Frontiers in Psychiatry. 4. Frontiers Media SA: 1. doi:10.3389/fpsyt.2013.00001. PMC 3550518. PMID 23346060.
- ^ "Can Dronabinol Help Treat Sleep Apnea? | HealthCentral". www.healthcentral.com. 25 April 2018. Retrieved 2018-11-04.
- ^ Carley DW, Prasad B, Reid KJ, Malkani R, Attarian H, Abbott SM, et al. (January 2018). "Pharmacotherapy of Apnea by Cannabimimetic Enhancement, the PACE Clinical Trial: Effects of Dronabinol in Obstructive Sleep Apnea". Sleep. 41 (1). doi:10.1093/sleep/zsx184. PMC 5806568. PMID 29121334.
- ^ "Drug Dronabinol Reduces Symptoms of Obstructive Sleep Apnea, Finds Phase 2B Study". Sleep Review. Retrieved 2018-11-04.
- ^ "Synthetic Cannabis-Like Drug Reduces Sleep Apnea". Neuroscience News. 2017-11-29. Retrieved 2018-11-04.
- ^ Carley DW, Prasad B, Reid KJ, Malkani R, Attarian H, Abbott S, et al. (2017-04-28). "0558 Dronabinol Reduces Ahi and Daytime Sleepiness in Patients with Moderate to Severe Obstructive Sleep Apnea Syndrome". Journal of Sleep and Sleep Disorders Research. 40 (suppl_1): A207 – A208. doi:10.1093/sleepj/zsx050.557. ISSN 0161-8105.
- ^ "HIGHLIGHTS OF PRESCRIBING INFORMATION: MARINOL (dronabinol) capsules, for oral use, CIII Initial U.S. Approval: 1985" (PDF). Food and Drug Administration.
- ^ "Dronabinol capsule (American Health Packaging)". US National Library of Medicine. July 2012. Retrieved 12 January 2014.
- ^ a b "1999 - Rescheduling of the Food and Drug Administration Approved Product Containing Synthetic Dronabinol [(-)-D9-(trans)-Tetrahydrocannabinol] in Sesame Oil and Encapsulated in Soft Gelatin Capsules From Schedule II to Schedule III". DEA Diversion Control Division. 1999-07-02. Archived from the original on 2021-05-01. Retrieved 2021-05-19.
- ^ 51 Fed. Reg. 17476 (1986), Tuesday, May 13, 1986, pages 17476-17478
- ^ Riboulet-Zemouli K, Krawitz MA, Ghehiouèche F (2018). The Crimson Digest (Vol. 1), Briefing on the international scientific assessment of Cannabis: Processes, stakeholders and history. Paris: FAAAT. pp. 37–43. ISBN 979-10-97087-06-7.
- ^ Calhoun SR, Galloway GP, Smith DE (1998). "Abuse potential of dronabinol (Marinol)". Journal of Psychoactive Drugs. 30 (2): 187–96. doi:10.1080/02791072.1998.10399689. PMID 9692381.
- ^ "Petition to Reschedule Cannabis (Marijuana)" (PDF). Coalition for Rescheduling Cannabis. 9 October 2002. Archived from the original (PDF) on 8 April 2018. Retrieved 25 January 2018.
- ^ "WHO Expert Committee on Drug Dependence". World Health Organization. Archived from the original on January 7, 2005. Retrieved 12 January 2014.
- ^ Riboulet-Zemouli K, Krawitz MA, Ghehiouèche F (2021). "History, Science, and Politics of International Cannabis Scheduling, 2015–2021". FAAAT editions. Rochester, NY. SSRN 3932639 – via SSRN.
- ^ EMCDDA, ELDD Comparative Study, May 2002.
- ^ "Marinol – the Legal Medical Use for the Marijuana Plant". Drug Enforcement Administration. Archived from the original on 21 October 2002. Retrieved 20 April 2011.
- ^ "Marijuana and Medicine: Cesamet, Marinol, Sativex". Alchimia Blog. Archived from the original on 20 February 2016.
- ^ "Tetra Bio-Pharma Files New Drug Submission for REDUVO™ in Canada". AP News. 30 December 2020.
- ^ "Tetra Bio-Pharma Hits Another Milestone Before Year End: Inhaled Dronabinol & MucoAdhesive Dronabinol 'Adversa'". Tetra Bio-Pharma. 16 December 2020. Archived from the original on 26 November 2020. Retrieved 2 January 2021.
- ^ Downs D (21 October 2014). "War on marijuana unconstitutional, doctors testify in federal court Monday". sfgate.com. Archived from the original on 22 October 2014. Retrieved 21 October 2014.
- ^ Eustice C (12 August 1997). "Medicinal Marijuana: A Continuing Controversy". About.com. Archived from the original on 15 June 2011. Retrieved 20 April 2011.
- ^ Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–31. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ^ Pickens JT (April 1981). "Sedative activity of cannabis in relation to its delta'-trans-tetrahydrocannabinol and cannabidiol content". British Journal of Pharmacology. 72 (4): 649–56. doi:10.1111/j.1476-5381.1981.tb09145.x. PMC 2071638. PMID 6269680.
- ^ Burns TL, Ineck JR (February 2006). "Cannabinoid analgesia as a potential new therapeutic option in the treatment of chronic pain". The Annals of Pharmacotherapy. 40 (2): 251–60. doi:10.1345/aph.1G217. PMID 16449552. S2CID 6858360.
- ^ MARINOL (dronabinol) capsule drug label/data at DailyMed from U.S. National Library of Medicine, National Institutes of Health.
- ^ McKim WA (2002). Drugs and Behavior: An Introduction to Behavioral Pharmacology (5th ed.). Prentice Hall. p. 400. ISBN 978-0-13-048118-4.
- ^ Greenberg G (1 November 2005). "Respectable Reefer". Mother Jones. Retrieved 8 April 2010.
- ^ "Cannabis and Cannabinoids (PDQ)". Cancer Topics. National Cancer Institute, U.S. Department of Health and Human Services. 2011-03-16.
- ^ "Government eases restrictions on pot derivative". Online Athens. Archived from the original on 2014-12-16. Retrieved 12 January 2014.
- ^ "21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I." Archived from the original on 2009-08-27. Retrieved 2021-01-10.
