Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a type of progressive lung disease characterized by chronic respiratory symptoms and airflow limitation.[8] GOLD 2024 defined COPD as a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea or shortness of breath, cough, sputum production and/or exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction.[9]
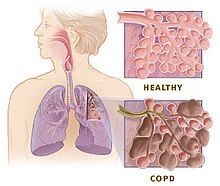
The main symptoms of COPD include shortness of breath and a cough, which may or may not produce mucus.[4] COPD progressively worsens, with everyday activities such as walking or dressing becoming difficult.[3] While COPD is incurable, it is preventable and treatable. The two most common types of COPD are emphysema and chronic bronchitis and have been the two classic COPD phenotypes. However, this basic dogma has been challenged as varying degrees of co-existing emphysema, chronic bronchitis, and potentially significant vascular diseases have all been acknowledged in those with COPD, giving rise to the classification of other phenotypes or subtypes.[10]
Emphysema is defined as enlarged airspaces (alveoli) whose walls have broken down resulting in permanent damage to the lung tissue. Chronic bronchitis is defined as a productive cough that is present for at least three months each year for two years. Both of these conditions can exist without airflow limitation when they are not classed as COPD. Emphysema is just one of the structural abnormalities that can limit airflow and can exist without airflow limitation in a significant number of people.[11][12] Chronic bronchitis does not always result in airflow limitation. However, in young adults with chronic bronchitis who smoke, the risk of developing COPD is high.[13] Many definitions of COPD in the past included emphysema and chronic bronchitis, but these have never been included in GOLD report definitions.[14] Emphysema and chronic bronchitis remain the predominant phenotypes of COPD but there is often overlap between them and a number of other phenotypes have also been described.[10][15] COPD and asthma may coexist and converge in some individuals.[16] COPD is associated with low-grade systemic inflammation.[17]
The most common cause of COPD is tobacco smoking.[18] Other risk factors include indoor and outdoor air pollution including dust, exposure to occupational irritants such as dust from grains, cadmium dust or fumes, and genetics, such as alpha-1 antitrypsin deficiency.[13][19] In developing countries, common sources of household air pollution are the use of coal and biomass such as wood and dry dung as fuel for cooking and heating.[20][13] The diagnosis is based on poor airflow as measured by spirometry.[4]
Most cases of COPD can be prevented by reducing exposure to risk factors such as smoking and indoor and outdoor pollutants.[21] While treatment can slow worsening, there is no conclusive evidence that any medications can change the long-term decline in lung function.[6] COPD treatments include smoking cessation, vaccinations, pulmonary rehabilitation, inhaled bronchodilators and corticosteroids.[6] Some people may benefit from long-term oxygen therapy, lung volume reduction and lung transplantation.[22] In those who have periods of acute worsening, increased use of medications, antibiotics, corticosteroids and hospitalization may be needed.[23]
As of 2015, COPD affected about 174.5 million people (2.4% of the global population).[7] It typically occurs in males and females over the age of 35–40.[1][3] In 2019 it caused 3.2 million deaths, 80% occurring in lower and middle income countries,[3] up from 2.4 million deaths in 1990.[24][25] In 2021, it was the fourth biggest cause of death, responsible for approximately 5% of total deaths.[26] The number of deaths is projected to increase further because of continued exposure to risk factors and an aging population.[14] In the United States in 2010 the economic cost was put at US$32.1 billion and projected to rise to US$49 billion in 2020.[27] In the United Kingdom this cost is estimated at £3.8 billion annually.[28]
Signs and symptoms
[edit]
Shortness of breath
[edit]A cardinal symptom of COPD is the chronic and progressive shortness of breath which is most characteristic of the condition. Shortness of breath (breathlessness) is often the most distressing symptom responsible for the associated anxiety and level of disability experienced.[4] Symptoms of wheezing and chest tightness associated with breathlessness can be variable over the course of a day or between days and are not always present. Chest tightness often follows exertion.[4] Many people with more advanced COPD breathe through pursed lips, which can improve shortness of breath.[29] Shortness of breath is often responsible for reduced physical activity and low levels of physical activity are associated with worse outcomes.[30][31] In severe and very severe cases there may be constant tiredness, weight loss, muscle loss and anorexia. People with COPD often have increased breathlessness and frequent colds before seeking treatment.[4]
Cough
[edit]The most often first symptom of COPD is a chronic cough, which may or may not be productive of mucus as phlegm. Phlegm coughed up as sputum can be intermittent and may be swallowed or spat out depending on social or cultural factors and is therefore not always easy to evaluate. However, an accompanying productive cough is only seen in up to 30% of cases. Sometimes limited airflow may develop in the absence of a cough.[4] Symptoms are usually worse in the morning.[32]
A chronic productive cough is the result of mucus hypersecretion and when it persists for more than three months each year for at least two years, it is defined as chronic bronchitis.[13] Chronic bronchitis can occur before the restricted airflow diagnostic of COPD.[14] Some people with COPD attribute the symptoms to the consequences of smoking. In severe COPD, vigorous coughing may lead to rib fractures or to a brief loss of consciousness.[4]
Exacerbations
[edit]An acute exacerbation is a sudden worsening of signs and symptoms that lasts for several days. The key symptom is increased breathlessness, other more pronounced symptoms are of excessive mucus, increased cough and wheeze. A commonly found sign is air trapping giving a difficulty in complete exhalation.[33] The usual cause of an exacerbation is a viral infection, most often the common cold.[13] The common cold is usually associated with the winter months but can occur at any time.[34] Other respiratory infections may be bacterial or in combination sometimes secondary to a viral infection.[35] The most common bacterial infection is caused by Haemophilus influenzae.[36] Other risks include exposure to tobacco smoke (active and passive) and environmental pollutants – both indoor and outdoor.[37] During the COVID-19 pandemic, hospital admissions for COPD exacerbations sharply decreased which may be attributable to reduction of emissions and cleaner air.[38] There has also been a marked decrease in the number of cold and flu infections during this time.[39]
Smoke from wildfires is proving an increasing risk in many parts of the world and government agencies have published protective advice on their websites. In the US the EPA advises that the use of dust masks do not give protection from the fine particles in wildfires and instead advise the use of well-fitting particulate masks.[40] This same advice is offered in Canada and Australia to the effects of their forest fires.[41][42]
The number of exacerbations is not seen to relate to any stage of the disease; those with two or more a year are classed as frequent exacerbators and these lead to a worsening in the disease progression.[33] Frailty in ageing increases exacerbations and hospitalization.[43]
Acute exacerbations in COPD are often unexplained and thought to have many causes other than infections. A study has emphasized the possibility of a pulmonary embolism as sometimes being responsible in these cases. Signs can include pleuritic chest pain and heart failure without signs of infection. Such emboli could respond to anticoagulants.[44]
Other conditions
[edit]COPD often occurs along with a number of other conditions (comorbidities) due in part to shared risk factors. Common comorbidities include cardiovascular disease, skeletal muscle dysfunction, metabolic syndrome, osteoporosis, depression, anxiety, asthma and lung cancer.[45] Alpha-1 antitrypsin deficiency (A1AD) is an important risk factor for COPD.[46] It is advised that everybody with COPD be screened for A1AD.[47] Metabolic syndrome has been seen to affect up to fifty percent of those with COPD and significantly affects the outcomes.[48] When comorbid with COPD there is more systemic inflammation.[48] It is not known if it co-exists with COPD or develops as a consequence of the pathology. Metabolic syndrome on its own has a high rate of morbidity and mortality and this rate is amplified when comorbid with COPD. Tuberculosis is a risk factor for the development of COPD, and is also a potential comorbidity.[13] Most people with COPD die from comorbidities and not from respiratory problems.[49]
Anxiety and depression are often complications of COPD.[2][1] Other complications include reduced quality of life and increased disability, cor pulmonale, frequent chest infections including pneumonia, secondary polycythemia, respiratory failure, pneumothorax, lung cancer, and cachexia (muscle wasting).[1][2][50]
Along with these complications, there is an associated risk of developing pulmonary hypertension. The estimated prevalence of pulmonary hypertension complicating COPD was reported at 39% in a meta-analysis.[51] Of the people with COPD listed for lung transplantation, 82% were documented as having pulmonary hypertension via right heart catheterization, noting a mean pulmonary arterial pressure greater than 20mm Hg.[51] Despite pulmonary hypertension being relatively rare in people with COPD, mild elevations of pulmonary arterial pressure can lead to worse outcomes, including risk of death.[51]
Cognitive impairment is common in those with COPD as it is for other lung conditions that affect airflow. Cognitive impairment is associated with the declining ability to cope with the basic activities of daily living.[52]
It is unclear if those with COPD are at greater risk of contracting COVID-19, though if infected they are at risk of hospitalization and developing severe COVID-19. However, there are laboratory and clinical studies showing a possibility of certain inhaled corticosteroids for COPD providing a protective role against COVID-19.[53] Differentiating COVID-19 symptoms from an exacerbation is difficult; mild prodromal symptoms may delay its recognition and where they include loss of taste or smell COVID-19 is to be suspected.[38]
Definition
[edit]Many definitions of COPD in the past included chronic bronchitis and emphysema but these have never been included in GOLD report definitions.[14] Emphysema is defined as enlarged airspaces (alveoli) whose walls break down resulting in permanent damage to the lung tissue and is just one of the structural abnormalities that can limit airflow. The condition can exist without airflow limitation but commonly it does.[11] Chronic bronchitis is defined as a productive cough that is present for at least three months each year for two years but does not always result in airflow limitation although the risk of developing COPD is great.[13] These older definitions grouped the two types as type A and type B. Type A were emphysema types known as pink puffers due to their pink complexion, fast breathing rate and pursed lips. Type B were chronic bronchitic types referred to as blue bloaters due to low oxygen levels causing a bluish color to the skin and lips and swollen ankles.[54] These differences were suggested to be due to the presence or not of collateral ventilation, evident in emphysema and lacking in chronic bronchitis.[55] This terminology was no longer accepted as useful, as most people with COPD have a combination of both emphysema and airway disease.[54] These are now recognized as the two major phenotypes of COPD — emphysematous phenotype and chronic bronchitic phenotype.[10]
Subtypes
[edit]It has since been recognized that COPD is more complex, with a diverse group of disorders of differing risk factors and clinical courses that has resulted in a number of subtypes or phenotypes of COPD being accepted and proposed.[56][57] The two classic emphysematous and chronic bronchitic phenotypes are fundamentally different conditions with unique underlying mechanisms.[10] Another subtype of COPD, categorized by some as a separate clinical entity, is asthma-COPD overlap, which is a condition sharing clinical features of both asthma and COPD.[58][59] Spirometry measures are inadequate for defining phenotypes and chest X-ray, CT and MRI scans have been mostly employed. Most cases of COPD are diagnosed at a late stage and the use of imaging methods would allow earlier detection and treatment.[10]
The identification and recognition of different phenotypes can guide appropriate treatment approaches. For example, the PDE4 inhibitor roflumilast is targeted at the chronic-bronchitic phenotype.[60]
Two inflammatory phenotypes show a phenotype stability: the neutrophilic inflammatory phenotype and the eosinophilic inflammatory phenotype.[61] Mepolizumab, a monoclonal antibody, has been shown to have benefit in treating the eosinophilic inflammatory type rather than the use of oral corticosteroids, but further studies have been called for.[62]
Another recognized phenotype is the frequent exacerbator.[63] The frequent exacerbator has two or more exacerbations a year, has a poor prognosis and is described as a moderately stable phenotype.[33]
A pulmonary vascular COPD phenotype has been described due to cardiovascular dysfunction.[64] A molecular phenotype of CFTR dysfunction is shared with cystic fibrosis.[15] A combined phenotype of chronic bronchitis and bronchiectasis has been described with a difficulty noted of determining the best treatment.[65]
The only genotype is the alpha-1 antitrypsin deficiency (AATD) genetic subtype and this has a specific treatment.[66]
Cause
[edit]The cause of the development of COPD is the exposure to harmful particles or gases, including tobacco smoke, that irritate the lung causing inflammation that interacts with a number of host factors. Such exposure needs to be significant or long-term.[14] The greatest risk factor for the development of COPD is tobacco smoke.[18] However, less than 50 percent of heavy smokers develop COPD, so other factors need to be considered, including exposure to indoor and outdoor pollutants, allergens, occupational exposure, and host factors.[32][13] One of the known causes of COPD is the exposure to construction dust. The three main types of construction dust are silica dust, non-silica dust (e.g., dust from gypsum, cement, limestone, marble and dolomite) and wood dust.[67] Host factors include a genetic susceptibility, factors associated with poverty, aging and physical inactivity. Asthma and tuberculosis are also recognized as risk factors, as the comorbidity of COPD is reported to be 12 times higher in patients with asthma after adjusting for smoking history.[13] In Europe airway hyperresponsiveness is rated as the second most important risk factor after smoking.[13]
A host factor of an airway branching variation, arising during development has been described.[68] The respiratory tree is a filter for harmful substances and any variant has the potential to disrupt this. A variation has been found to be associated with the development of chronic bronchitis and another with the development of emphysema. A branch variant in the central airway is specifically associated with an increased susceptibility for the later development of COPD. A genetic association for the variants has been sometimes found with FGF10.[68][69]
Alcohol abuse can lead to alcoholic lung disease and is seen to be an independent risk factor for COPD.[70][71] Mucociliary clearance is disrupted by chronic exposure to alcohol; macrophage activity is diminished and an inflammatory response promoted.[72][71] The damage leads to a susceptibility for infection, including COVID-19,[73] more so when combined with smoking; smoking induces the upregulation of the expression of ACE2, a receptor for the SARS-CoV-2 virus.[70]
Smoking
[edit]The primary risk factor for COPD globally is tobacco smoking with an increased rate of developing COPD shown in smokers and ex-smokers.[14][18] Of those who smoke, about 20% will get COPD,[74] increasing to less than 50% in heavy smokers.[14] In the United States and United Kingdom, of those with COPD, 80–95% are either current or previous smokers.[74][75][76] Several studies indicate that women are more susceptible than men to the harmful effects of tobacco smoke.[77] For the same amount of cigarette smoking, women have a higher risk of COPD than men.[78] In non-smokers, exposure to second-hand smoke (passive smoking) is the cause of 1.2 million deaths from the more than 8 million deaths worldwide each year due to tobacco smoke.[79] Women who smoke during pregnancy, and during the early life of the child is a risk factor for the later development of COPD in their child.[80]
Inhaled smoke triggers the release of excessive proteases in lungs, which then degrades elastin, the major component of alveoli.[18] Smoke also impairs the action of cilia, inhibiting mucociliary clearance that clears the bronchi of mucus, cellular debris and unwanted fluid.[18]
Other types of tobacco smoke, such as from cigar, pipe, water-pipe and hookah use, also confer a risk.[13] Water-pipe or hookah smoke appears to be as harmful or even more harmful than smoking cigarettes.[81]
Marijuana is the second most commonly smoked substance, but evidence linking its use to COPD is very limited. Limited evidence shows that marijuana does not accelerate lung function decline.[82] A low use of marijuana gives a bronchodilatory effect rather than the bronchoconstrictive effect from tobacco use, but it is often smoked in combination with tobacco or on its own by tobacco smokers. Higher use however has shown a decline in the FEV1.[83] There is evidence of it causing some respiratory problems and its use in combination may have a cumulative toxic effect suggesting it as a risk factor for spontaneous pneumothorax, bullous emphysema, COPD and lung cancer.[82][84] A noted difference between marijuana use and tobacco was that respiratory problems were resolved with stopping usage unlike the continued decline with stopping tobacco smoking.[82] Respiratory symptoms reported with marijuana use included chronic cough, increased sputum production and wheezing but not shortness of breath. Also these symptoms were typically reported ten years ahead of their affecting tobacco smokers.[82] Another study found that chronic marijuana smokers even with the additional use of tobacco developed similar respiratory problems, but did not seem to develop airflow limitation and COPD.[85]
Pollution
[edit]
Exposure to particulates can bring about the development of COPD, or its exacerbations. Those with COPD are more susceptible to the harmful effects of particulate exposure that can cause acute exacerbations brought about by infections.[48] Black carbon also known as soot, is an air pollutant associated with an increased risk of hospitalization due to the exacerbations caused. Long-term exposure is indicated as an increased rate of mortality in COPD.[48] Studies have shown that people who live in large cities have a higher rate of COPD compared to people who live in rural areas.[87] Areas with poor outdoor air quality, including that from exhaust gas, generally have higher rates of COPD.[88] Urban air pollution significantly effects the developing lung and its maturation, and contributes a potential risk factor for the later development of COPD. The overall effect in relation to smoking is believed to be small.[13]
Poorly ventilated fires used for cooking and heating, are often fueled by coal or biomass such as wood and dry dung, leading to indoor air pollution and are one of the most common causes of COPD in developing countries. Women are affected more as they have a greater exposure.[13] These fuels are used as the main source of energy in 80% of homes in India, China and sub-Saharan Africa.[88]
Occupational exposure
[edit]Intense and prolonged exposure to workplace dusts, chemicals and fumes increases the risk of COPD in smokers, nonsmokers and never-smokers. Substances implicated in occupational exposure and listed in the UK, include organic and inorganic dusts such as cadmium, silica, dust from grains and flour and fumes from cadmium and welding that promote respiratory symptoms.[19][13] Workplace exposure is believed to be the cause in 10–20% of cases and in the United States, it is believed to be related to around 30% of cases among never smokers and probably represents a greater risk in countries without sufficient regulations.[13][89] The negative effects of dust exposure and cigarette smoke exposure appear to be cumulative.[90]
Genetics
[edit]Genetics play a role in the development of COPD. It is more common among relatives of those with COPD who smoke than unrelated smokers.[13] The most well known genetic risk factor is alpha-1 antitrypsin deficiency (AATD) and this is the only genotype (genetic subtype) with a specific treatment.[66] This risk is particularly high if someone deficient in alpha-1 antitrypsin (AAT) also smokes.[91] It is responsible for about 1–5% of cases[91][92] and the condition is present in about three to four in 10,000 people.[93]
Mutations in MMP1 gene that encodes for interstitial collagenase are associated with COPD.[94]
The COPDGene study is an ongoing longitudinal study into the epidemiology of COPD, identifying phenotypes and looking for their likely association with susceptible genes. Genome wide analyses in concert with the International COPD Genetics Consortium has identified more than 80 genome regions associated with COPD and further studies in these regions has been called for. Whole genome sequencing is an ongoing collaboration (2019) with the National Heart, Lung and Blood Institute (NHLBI) to identify rare genetic determinants.[95]
Pathophysiology
[edit]
COPD is a progressive lung disease in which chronic, incompletely reversible poor airflow (airflow limitation) and an inability to breathe out fully (air trapping) exist.[96] The poor airflow is the result of small airways disease and emphysema (the breakdown of lung tissue).[97] The relative contributions of these two factors vary between people.[14] Air trapping precedes lung hyperinflation.[98]
COPD develops as a significant and chronic inflammatory response to inhaled irritants which ultimately leads to bronchial and alveolar remodelling in the lung known as small airways disease.[99][100][101] Thus, airway remodelling with narrowing of peripheral airway and emphysema are responsible for the alteration of lung function.[61] Mucociliary clearance is particularly altered with a dysregulation of cilia and mucus production.[102] Small airway disease sometimes called chronic bronchiolitis, appears to be the precursor for the development of emphysema.[103] The inflammatory cells involved include neutrophils and macrophages, two types of white blood cells. Those who smoke additionally have cytotoxic T cell involvement and some people with COPD have eosinophil involvement similar to that in asthma. Part of this cell response is brought on by inflammatory mediators such as chemotactic factors. Other processes involved with lung damage include oxidative stress produced by high concentrations of free radicals in tobacco smoke and released by inflammatory cells and breakdown of the connective tissue of the lungs by proteases (particularly elastase) that are insufficiently inhibited by protease inhibitors. The destruction of the connective tissue of the lungs leads to emphysema, which then contributes to the poor airflow and finally, poor absorption and release of respiratory gases. General muscle wasting that often occurs in COPD may be partly due to inflammatory mediators released by the lungs into the blood.[13]
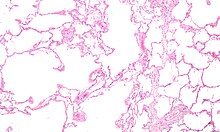
Narrowing of the airways occurs due to inflammation and subsequent scarring within them. This contributes to the inability to breathe out fully. The greatest reduction in air flow occurs when breathing out, as the pressure in the chest is compressing the airways at this time.[104] This can result in more air from the previous breath remaining within the lungs when the next breath is started, resulting in an increase in the total volume of air in the lungs at any given time, a process called air trapping which is closely followed by hyperinflation.[104][105][98] Hyperinflation from exercise is linked to shortness of breath in COPD, as breathing in is less comfortable when the lungs are already partly filled.[106] Hyperinflation may also worsen during an exacerbation.[107] There may also be a degree of airway hyperresponsiveness to irritants similar to those found in asthma.[93]
Low oxygen levels and eventually, high carbon dioxide levels in the blood, can occur from poor gas exchange due to decreased ventilation from airway obstruction, hyperinflation and a reduced desire to breathe.[13] During exacerbations, airway inflammation is also increased, resulting in increased hyperinflation, reduced expiratory airflow and worsening of gas transfer. This can lead to low blood oxygen levels which if present for a prolonged period, can result in narrowing of the arteries in the lungs, while emphysema leads to the breakdown of capillaries in the lungs. Both of these conditions may result in pulmonary heart disease also classically known as cor pulmonale.[50]
Diagnosis
[edit]
The diagnosis of COPD should be considered in anyone over the age of 35 to 40 who has shortness of breath, a chronic cough, sputum production, or frequent winter colds and a history of exposure to risk factors for the disease. Spirometry is then used to confirm the diagnosis.[4][108]
Spirometry
[edit]Spirometry measures the amount of airflow obstruction present and is generally carried out after the use of a bronchodilator, a medication to open up the airways.[109] Two main components are measured to make the diagnosis, the forced expiratory volume in one second (FEV1), which is the greatest volume of air that can be breathed out in the first second of a breath and the forced vital capacity (FVC), which is the greatest volume of air that can be breathed out in a single large breath.[110] Normally, 75–80% of the FVC comes out in the first second[110] and a FEV1/FVC ratio less than 70% in someone with symptoms of COPD defines a person as having the disease.[109] Based on these measurements, spirometry would lead to over-diagnosis of COPD in the elderly.[109] The National Institute for Health and Care Excellence criteria additionally require a FEV1 less than 80% of predicted.[111] People with COPD also exhibit a decrease in diffusing capacity of the lung for carbon monoxide due to decreased surface area in the alveoli, as well as damage to the capillary bed.[112] Testing the peak expiratory flow (the maximum speed of expiration), commonly used in asthma diagnosis, is not sufficient for the diagnosis of COPD.[111]
Screening using spirometry in those without symptoms has uncertain effect and is generally not recommended; however, it is recommended for those without symptoms but with a known risk factor.[45]
Assessment
[edit]| Grade | Activity affected |
|---|---|
| 1 | Only strenuous activity |
| 2 | Vigorous walking |
| 3 | With normal walking |
| 4 | After a few minutes of walking |
| 5 | With changing clothing |
| Severity | FEV1% predicted |
|---|---|
| Mild (GOLD 1) | ≥80 |
| Moderate (GOLD 2) | 50–79 |
| Severe (GOLD 3) | 30–49 |
| Very severe (GOLD 4) | <30 |
A number of methods can be used to assess the affects and severity of COPD.[108][45] The MRC breathlessness scale or the COPD assessment test (CAT) are simple questionnaires that may be used.[113][108] GOLD refers to a modified MRC scale that if used, needs to include other tests since it is simply a test of breathlessness experienced.[45][114] Scores on CAT range from 0–40 with the higher the score, the more severe the disease.[115] Spirometry may help to determine the severity of airflow limitation.[4] This is typically based on the FEV1 expressed as a percentage of the predicted "normal" for the person's age, gender, height and weight.[4] Guidelines published in 2011 by American and European medical societies recommend partly basing treatment recommendations on the FEV1.[109] The GOLD guidelines group people into four categories based on symptoms assessment, degree of airflow limitation and history of exacerbations.[114] Weight loss, muscle loss and fatigue are seen in severe and very severe cases.[45]
Use of screening questionnaires, such as COPD diagnostic questionnaire (CDQ), alone or in combination with hand-held flow meters is appropriate for screening of COPD in primary care.[116]
Other tests
[edit]A chest X-ray is not useful to establish a diagnosis of COPD but it is of use in either excluding other conditions or including comorbidities such as pulmonary fibrosis and bronchiectasis. Characteristic signs of COPD on X-ray include hyperinflation (shown by a flattened diaphragm and an increased retrosternal air space) and lung hyperlucency.[5] A saber-sheath trachea may also be shown that is indicative of COPD.[117]
A CT scan is not routinely used except for the exclusion of bronchiectasis.[5] An analysis of arterial blood is used to determine the need for oxygen supplementation and assess for high levels of carbon dioxide in the blood; this is recommended in those with an FEV1 less than 35% predicted, those with a peripheral oxygen saturation less than 92% and those with symptoms of congestive heart failure.[118] WHO recommends that all those diagnosed with COPD be screened for alpha-1 antitrypsin deficiency.[45]
-
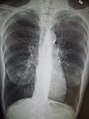
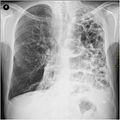
Chest X-ray demonstrating severe COPD, displaying small heart size in comparison to the lungs
-
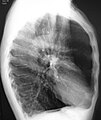
A lateral chest X-ray of a person with emphysema, displaying barrel chest and flat diaphragm
-
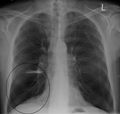
Lung bulla as seen on chest X-ray in a person with severe COPD
-
A severe case of bullous emphysema
-
Axial CT image of the lung of a person with end-stage bullous emphysema
-
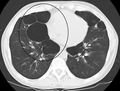
Very severe emphysema with lung cancer on the left (CT scan)
Differential diagnosis
[edit]COPD may need to be differentiated from other conditions such as congestive heart failure, asthma, bronchiectasis, tuberculosis, obliterative bronchiolitis and diffuse panbronchiolitis.[5] The distinction between asthma and COPD is made on the basis of the symptoms, smoking history and whether airflow limitation is reversible with bronchodilators at spirometry.[119] Chronic bronchitis with normal airflow is not classified as COPD.[93]
Prevention
[edit]Most cases of COPD are potentially preventable through decreasing exposure to tobacco smoke and other indoor and outdoor pollutants.[21]
Smoking cessation
[edit]The policies of governments, public health agencies and antismoking organizations can reduce smoking rates by discouraging people from starting and encouraging people to stop smoking.[120] Smoking bans in public areas and places of work are important measures to decrease exposure to secondhand smoke and while many places have instituted bans, more are recommended.[88]
In those who smoke, stopping smoking is the only measure shown to slow down the worsening of COPD.[121][122] Even at a late stage of the disease, it can reduce the rate of worsening lung function and delay the onset of disability and death.[123] Often, several attempts are required before long-term abstinence is achieved.[120] Attempts over 5 years lead to success in nearly 40% of people.[124]
Some smokers can achieve long-term smoking cessation through willpower alone. Smoking, however, is highly addictive and many smokers need further support.[125] The chance of quitting is improved with social support, engagement in a smoking cessation program and the use of medications such as nicotine replacement therapy, bupropion, or varenicline.[120][122][124] Combining smoking-cessation medication with behavioral therapy is more than twice as likely to be effective in helping people with COPD stop smoking, compared with behavioral therapy alone.[126]
Occupational health
[edit]A number of measures have been taken to reduce the likelihood that workers in at-risk industries—such as coal mining, construction and stonemasonry—will develop COPD.[88] Examples of these measures include the creation of public policy,[88] education of workers and management about the risks, promoting smoking cessation, checking workers for early signs of COPD, use of respirators and dust control.[127][128] Effective dust control can be achieved by improving ventilation, using water sprays and by using mining techniques that minimize dust generation.[129] If a worker develops COPD, further lung damage can be reduced by avoiding ongoing dust exposure, for example by changing their work role.[130]
Pollution control
[edit]Both indoor and outdoor air quality can be improved, which may prevent COPD or slow the worsening of existing disease.[88] This may be achieved by public policy efforts, cultural changes and personal involvement.[131] Many developed countries have successfully improved outdoor air quality through regulations which has resulted in improvements in the lung function of their populations.[88] Individuals are also advised to avoid irritants of indoor and outdoor pollution.[21]
In developing countries one key effort is to reduce exposure to smoke from cooking and heating fuels through improved ventilation of homes and better stoves and chimneys.[131] Proper stoves may improve indoor air quality by 85%. Using alternative energy sources such as solar cooking and electrical heating is also effective. Using fuels such as kerosene or coal might produce less household particulate matter than traditional biomass such as wood or dung, but whether this is better health wise is unclear.[88]
Management
[edit]COPD currently has no cure,[132] but the symptoms are treatable and its progression can be delayed, particularly by stopping smoking.[1][6] The major goals of management are to reduce exposure to risk factors including offering non-pharmacological treatments such as help with stopping smoking. Stopping smoking can reduce the rate of lung function decline and also reduce mortality from smoking-related diseases such as lung cancer and cardiovascular disease.[1] Other recommendations include annual influenza vaccinations and pneumococcal vaccination to help reduce the risk of exacerbations; CDC and GOLD 2024 also recommends RSV vaccine for individuals above 60 years;[133][134] giving advice as to healthy eating and encouraging physical exercise. Guidance is also advised as to managing breathlessness and stress.[6]
Other illnesses are also managed. An action plan is drawn up and is to be reviewed.[21] Providing people with a personalized action plan, an educational session and support for use of their action plan in the event of an exacerbation, reduces the number of hospital visits and encourages early treatment of exacerbations.[135] When self-management interventions, such as taking corticosteroids and using supplemental oxygen, is combined with action plans, health-related quality of life is improved compared to usual care.[136] In those with COPD who are malnourished, supplementation with vitamin C, vitamin E, zinc and selenium can improve weight, strength of respiratory muscles and health-related quality of life.[22] Significant vitamin D deficiency is common in those with COPD and can cause increased exacerbations. Supplementation when deficient can give a 50% reduction in the number of exacerbations.[33][137]
A number of medical treatments are used in the management of stable COPD and exacerbations. These include bronchodilators, corticosteroids and antibiotics.
In those with a severe exacerbation, antibiotics improve outcomes.[138] A number of different antibiotics may be used including amoxicillin, doxycycline and azithromycin; whether one is better than the others is unclear.[139] There is no clear evidence of improved outcomes for those with less severe cases.[138] The FDA recommends against the use of fluoroquinolones when other options are available due to higher risks of serious side effects.[140] In treating acute hypercapnic respiratory failure (acutely raised levels of carbon dioxide), bilevel positive airway pressure (BPAP) can decrease mortality and the need of intensive care.[141] Fewer than 20% of exacerbations require hospital admission.[131] In those without acidosis from respiratory failure, home care may be able to help avoid some admissions.[131]
In those with end-stage disease, palliative care is focused on relieving symptoms.[142] Morphine can improve exercise tolerance.[22] Non-invasive ventilation may be used to support breathing and also reduce daytime breathlessness.[143][22]
Bronchodilators
[edit]Inhaled short-acting bronchodilators are the primary medications used on an as needed basis; their use on a regular basis is not recommended.[6] The two major types are beta2-adrenergic agonists and anticholinergics; either in long-acting or short-acting forms. Beta2–adrenergic agonists target receptors in the smooth muscle cells in bronchioles causing them to relax and allow improved airflow. They reduce shortness of breath, tend to reduce dynamic hyperinflation and improve exercise tolerance.[6][144] Short-acting bronchodilators have an effect for four hours and for maintenance therapy long acting bronchodilators with an effect of over twelve hours are used. In times of more severe symptoms a short acting agent may be used in combination.[6] An inhaled corticosteroid used with a long-acting beta-2 agonist is more effective than either one on its own.[145]
Which type of long-acting agent, long-acting muscarinic antagonist (LAMA) such as tiotropium or long-acting beta agonist (LABA), is better is unclear and trying each and continuing with the one that works best may be advisable.[146] Both types of agent appear to reduce the risk of acute exacerbations by 15–25%.[141] The combination of LABA/LAMA may reduce COPD exacerbations and improve quality-of-life compared to long-acting bronchodilators alone.[147] The 2018 NICE guideline recommends use of dual long-acting bronchodilators with economic modelling suggesting that this approach is preferable to starting one long acting bronchodilator and adding another later.[148]
Several short-acting β2 agonists are available, including salbutamol (albuterol) and terbutaline.[131] They provide some relief of symptoms for four to six hours.[131] A long-acting beta agonist (LABA) such as salmeterol, formoterol and indacaterol are often used as maintenance therapy. Some feel the evidence of benefits is limited,[149] while others view the evidence of benefit as established.[150][151][152] Long-term use appears safe in COPD[153] with adverse effects include shakiness and heart palpitations.[141] When used with inhaled steroids they increase the risk of pneumonia.[141] While steroids and LABAs may work better together,[149] it is unclear if this slight benefit outweighs the increased risks.[154] There is some evidence that combined treatment of LABAs with long-acting muscarinic antagonists (LAMA), an anticholinergic, and LABA +ICS (inhaled corticosteroid) may be similar in benefits in terms of fewer exacerbation's and quality of life measures for moderate to severe COPD, but LAMA+LABA offers better improvements in forced expiratory volume (FEV1%) and a lower risk of pneumonia.[155] All three together, LABA, LAMA and ICS, have some evidence of benefits.[156] Indacaterol requires an inhaled dose once a day and is as effective as the other long-acting β2 agonist drugs that require twice-daily dosing for people with stable COPD.[152]
The two main anticholinergics used in COPD are ipratropium and tiotropium. Ipratropium is a short-acting muscarinic antagonist (SAMA), while tiotropium is long-acting (LAMA). Tiotropium is associated with a decrease in exacerbations and improved quality of life,[157] and tiotropium provides those benefits better than ipratropium.[158] It does not appear to affect mortality or the overall hospitalization rate.[157] Anticholinergics can cause dry mouth and urinary tract symptoms.[141] They are also associated with increased risk of heart disease and stroke.[159] Aclidinium, another long-acting agent, reduces hospitalizations associated with COPD and improves quality of life.[160][161][162] The LAMA umeclidinium bromide is another anticholinergic alternative.[163] When compared to tiotropium, the LAMAs aclidinium, glycopyrronium, and umeclidinium appear to have a similar level of efficacy; with all four being more effective than placebo.[164] Further research is needed comparing aclidinium to tiotropium.[162]
Corticosteroids
[edit]Inhaled corticosteroids are anti-inflammatories that are recommended by GOLD as a first-line maintenance treatment in COPD cases with repeated exacerbations.[165][166] Their regular use increases the risk of pneumonia in severe cases.[33] Studies have shown that the risk of pneumonia is associated with all types of corticosteroids; is related to the disease severity and a dose-response relationship has been noted.[165] Oral glucocorticoids can be effective in treating an acute exacerbation.[145] They appear to have fewer side effects than those given intravenously.[167] Five days of steroids work as well as ten or fourteen days.[168]
The use of corticosteroids is associated with a decrease in the number of lymphoid follicles (in the bronchial lymphoid tissue).[103] A triple inhaled therapy of LABA/LAMA/ICS improves lung function, reduces symptoms and exacerbations and is seen to be more effective than mono or dual therapies.[169][145] NICE guidelines recommend the use of ICSs in people with asthmatic features or features suggesting steroid responsiveness.[148]
PDE4 inhibitors
[edit]Phosphodiesterase-4 inhibitors (PDE4 inhibitors) are anti-inflammatories that improve lung function and reduce exacerbations in moderate to severe illness. Roflumilast is a PDE4 inhibitor used orally once daily to reduce inflammation, it has no direct bronchodilatory effects. It is essentially used in treating those with chronic bronchitis along with systemic corticosteroids.[62] Reported adverse effects of roflumilast appear early in treatment, become less with continued treatment and are reversible. One effect is dramatic weight loss and its use is to be avoided in underweight people. It is also advised to be used with caution in those who have depression.[62]
Other medications
[edit]Long-term preventive use of antibiotics, specifically those from the macrolide class such as erythromycin, reduce the frequency of exacerbations in those who have two or more a year.[170][171] This practice may be cost effective in some areas of the world.[172] Concerns include the potential for antibiotic resistance and side effects including hearing loss, tinnitus and changes to the heart rhythm known as long QT syndrome.[171]
Methylxanthines such as theophylline are widely used. Theophylline is seen to have a mild bronchodilatory effect in stable COPD. Inspiratory muscle function is seen to be improved but the causal effect is unclear. Theophylline is seen to improve breathlessness when used as an add-on to salmeterol. All instances of improvement have been reported using sustained release preparations.[6] Methylxanthines are not recommended for use in exacerbations due to adverse effects.[33]
Mucolytics may help to reduce exacerbations in some people with chronic bronchitis; noticed by less hospitalization and less days of disability in one month.[173] Erdosteine is recommended by NICE.[174] GOLD also supports the use of some mucolytics that are advised against when inhaled corticosteroids are being used and singles out erdosteine as having good effects regardless of corticosteroid use. Erdosteine also has antioxidant properties but there is not enough evidence to support the general use of antioxidants.[62] Erdosteine has been shown to significantly reduce the risk of exacerbations, shorten their duration and hospital stays.[175]
Cough medicines are not recommended.[176] Beta blockers are not contraindicated for those with COPD and should only be used where there is concomitant cardiovascular disease.[62]
Recent studies show that metformin plays a role in reducing systemic inflammation by reducing biomarker levels that are increased during COPD exacerbations.[177]
Oxygen therapy
[edit]Supplemental oxygen is recommended for those with low oxygen levels in respiratory failure at rest (a partial pressure of oxygen less than 50–55 mmHg or oxygen saturations of less than 88%).[22] When taking into account complications including cor pulmonale and pulmonary hypertension, the levels involved are 56–59 mmHg.[178] Oxygen therapy is to be used for between 15 and 18 hours per day and is said to decrease the risk of heart failure and death.[178] In those with normal or mildly low oxygen levels, oxygen supplementation (ambulatory) may improve shortness of breath when given during exercise, but may not improve breathlessness during normal daily activities or affect the quality of life.[179] During acute exacerbations, many require oxygen therapy; the use of high concentrations of oxygen without taking into account a person's oxygen saturations may lead to increased levels of carbon dioxide and worsened outcomes.[180][181] In those at high risk of high carbon dioxide levels, oxygen saturations of 88–92% are recommended, while for those without this risk, recommended levels are 94–98%.[181] Once prescribed long-term oxygen therapy, patients should be re-assessed after 60 to 90 days, to deteermine whether supplemental oxygen is still indicated and if prescribed supplemental oxygen is effective.[182][183]
Rehabilitation
[edit]Pulmonary rehabilitation is a program of exercise, disease management and counseling, coordinated to benefit the individual.[184] A severe exacerbation leads to hospital admission, high mortality and a decline in the ability to carry out daily activities. Following a hospital admission pulmonary rehabilitation has been shown to significantly reduce future hospital admissions, mortality and improve quality of life.[60]
The optimal exercise routine, use of noninvasive ventilation during exercise and intensity of exercise suggested for people with COPD, is unknown.[185][186] Performing endurance arm exercises improves arm movement for people with COPD and may result in a small improvement in breathlessness.[187] Performing arm exercises alone does not appear to improve quality of life.[187] Pursed-lip breathing exercises may be useful.[29] Tai chi exercises appear to be safe to practice for people with COPD and may be beneficial for pulmonary function and pulmonary capacity when compared to a regular treatment program.[188] Tai Chi was not found to be more effective than other exercise intervention programs.[188] Inspiratory and expiratory muscle training (IMT, EMT) have been suggested and may provide some improvements when compared to no treatment.[189] A combination of IMT and walking exercises at home may help limit breathlessness in cases of severe COPD.[190] Additionally, the use of low amplitude high velocity joint mobilization together with exercise improves lung function and exercise capacity.[191] The goal of spinal manipulation therapy is to improve thoracic mobility in an effort to reduce the work on the lungs during respiration, however, the evidence supporting manual therapy for people with COPD is very weak.[191][192]
Airway clearance techniques (ACTs), such as postural drainage, percussion/vibration, autogenic drainage, hand-held positive expiratory pressure (PEP) devices and other mechanical devices, may reduce the need for increased ventilatory assistance, the duration of ventilatory assistance and the length of hospital stay in people with acute COPD.[193] In people with stable COPD, ACTs may lead to short-term improvements in health-related quality of life and a reduced long-term need for hospitalizations related to respiratory issues.[193]
Being either underweight or overweight can affect the symptoms, degree of disability and prognosis of COPD. People with COPD who are underweight can improve their breathing muscle strength by increasing their calorie intake. When combined with regular exercise or a pulmonary rehabilitation program, this can lead to improvements in COPD symptoms. Supplemental nutrition may be useful in those who are malnourished.[22][194]
Management of exacerbations
[edit]People with COPD can experience exacerbations (flare-ups) that are commonly caused by respiratory tract infections. The symptoms that worsen are not specific to COPD and differential diagnoses need to be considered.[33] Acute exacerbations are typically treated by increasing the use of short-acting bronchodilators including a combination of a short-acting inhaled beta agonist and short-acting anticholinergic.[33] These medications can be given either via a metered-dose inhaler with a spacer or via a nebulizer, with both appearing to be equally effective.[131][195] Nebulization may be easier for those who are more unwell.[131] Oxygen supplementation can be useful. Excessive oxygen; however, can result in increased CO2 levels and a decreased level of consciousness.[196] Corticosteroids given orally can improve lung function and shorten hospital stays but their use is recommended for only five to seven days; longer courses increase the risk of pneumonia and death.[33]
Room temperature
[edit]Maintaining room temperature of at least 21 °C (70 °F) for a minimum of nine hours a day was associated with better health in those with COPD, especially for smokers.[197] The World Health Organization (WHO) recommends indoor temperatures of a slightly higher range between 18 and 24 °C (64 and 75 °F).[198]
Room humidity
[edit]For people with COPD, the ideal indoor humidity levels are 30–50% RH. Maintaining indoor humidity can be difficult in the winter, especially in cold climates where the heating system is constantly running.[199]
Keeping the indoor relative humidity above 40% RH significantly reduces the infectivity of aerosolized viruses.[200]
Procedures for emphysema
[edit]There are a number of procedures to reduce the volume of a lung in cases of severe emphysema with hyperinflation.
Surgical
[edit]For severe emphysema that has proved unresponsive to other therapies lung volume reduction surgery (LVRS) may be an option.[201][202] LVRS involves the removal of damaged tissue, which improves lung function by allowing the rest of the lungs to expand.[141][131] It is considered when the emphysema is in the upper lobes and when there are no comorbidities.[203]
Bronchoscopic
[edit]Minimally invasive bronchoscopic procedures may be carried out to reduce lung volume. These include the use of valves, coils, or thermal ablation.[22][204] Endobronchial valves are one-way valves that may be used in those with severe hyperinflation resulting from advanced emphysema; a suitable target lobe and no collateral ventilation are required for this procedure. The placement of one or more valves in the lobe induces a partial collapse of the lobe that ensures a reduction in residual volume that improves lung function, the capacity for exercise and quality of life.[205]
The placement of nitinol coils instead of valves is recommended where there is collateral ventilation that would prevent the use of valves.[206] Nitinol is a biocompatible alloy.
Both of these techniques are associated with adverse effects including persistent air leaks and cardiovascular complications. Thermal vapor ablation has an improved profile. Heated water vapor is used to target lobe regions which leads to permanent fibrosis and volume reduction. The procedure is able to target individual lobe segments, can be carried out regardless of collateral ventilation and can be repeated with the natural advance of emphysema.[207]
Other surgeries
[edit]In very severe cases lung transplantation might be considered.[201] A CT scan may be useful in surgery considerations.[93] Ventilation/perfusion scintigraphy is another imaging method that may be used to evaluate cases for surgical interventions and also to evaluate post-surgery responses.[208] A bullectomy may be carried out when a giant bulla occupies more than a third of a hemithorax.[203]
Prognosis
[edit]

| no data ≤110 110–220 220–330 330–440 440–550 550–660 | 660–770 770–880 880–990 990–1100 1100–1350 ≥1350 |
COPD is progressive and can lead to premature death. It is estimated that 3% of all disability is related to COPD.[210] The proportion of disability from COPD globally has decreased from 1990 to 2010 due to improved indoor air quality primarily in Asia.[210] The overall number of years lived with disability from COPD, however, has increased.[211]
There are many variables affecting the long-term outcome in COPD and GOLD recommends the use of a composite test (BODE) that includes the main variables of body-mass index, obstruction of airways, dyspnea (breathlessness) and exercise and not just spirometry results.[212]
NICE recommends against the use of BODE for the prognosis assessment in stable COPD; factors such as exacerbations and frailty need to be considered.[204] Other factors that contribute to a poor outcome include older age, comorbidities such as lung cancer and cardiovascular disease and the number and severity of exacerbations needing hospital admittance.[33]
Epidemiology
[edit]Estimates of prevalence have considerable variation due to differences in analytical and surveying approach and the choice of diagnostic criteria.[213] An estimated 384 million people aged 30 years or more had COPD in 2010, corresponding to a global prevalence of 12%.[14] The disease affects men and women.[3] The increase in the developing world between 1970 and the 2000s is believed to be related to increasing rates of smoking in this region, an increasing population and an aging population due to fewer deaths from other causes such as infectious diseases.[141] Some developed countries have seen increased rates, some have remained stable and some have seen a decrease in COPD prevalence.[141]
Around three million people die of COPD each year.[14] In some countries, mortality has decreased in men but increased in women.[214] This is most likely due to rates of smoking in women and men becoming more similar.[93] A higher rate of COPD is found in those over 40 years and this increases greatly with advancing age with the highest rate found in those over 60 years.[14] Sex differences in the anatomy of the respiratory system include smaller airway lumens and thicker airway walls in women, which contribute to a greater severity of COPD symptoms like dyspnea and frequency of COPD exacerbation.[215]
In the UK, three million people are reported to be affected by COPD – two million of these being undiagnosed. On average, the number of COPD-related deaths between 2007 and 2016 was 28,600. The estimated number of deaths due to occupational exposure was estimated to be about 15% at around 4,000.[213] In the United States in 2018, almost 15.7 million people had been diagnosed with COPD and it is estimated that millions more have not been diagnosed.[216]
In 2011, there were approximately 730,000 hospitalizations in the United States for COPD.[217] Globally, COPD in 2019 was the third-leading cause of death. In low-income countries, COPD does not appear in the Top 10 causes of death; in other income groups, it is in the Top 5.[218]
History
[edit]
The name chronic obstructive pulmonary disease is believed to have first been used in 1965.[219] Previously it has been known by a number of different names, including chronic obstructive bronchopulmonary disease, chronic airflow obstruction, chronic obstructive lung disease, nonspecific chronic pulmonary disease, diffuse obstructive pulmonary syndrome.[219]
The terms emphysema and chronic bronchitis were formally defined as components of COPD in 1959 at the CIBA guest symposium and in 1962 at the American Thoracic Society Committee meeting on Diagnostic Standards.[219]
Early descriptions of probable emphysema began in 1679 by T. Bonet of a condition of "voluminous lungs" and in 1769 by Giovanni Morgagni of lungs which were "turgid particularly from air".[219][220] In 1721 the first drawings of emphysema were made by Ruysh.[220] René Laennec, used the term emphysema in his book A Treatise on the Diseases of the Chest and of Mediate Auscultation (1837) to describe lungs that did not collapse when he opened the chest during an autopsy. He noted that they did not collapse as usual because they were full of air and the airways were filled with mucus.[219] In 1842, John Hutchinson invented the spirometer, which allowed the measurement of vital capacity of the lungs. However, his spirometer could only measure volume, not airflow. Tiffeneau and Pinelli in 1947 described the principles of measuring airflow.[219]
Air pollution and the increase in cigarette smoking in Great Britain at the start of the 20th century led to high rates of chronic lung disease, though it received little attention until the Great Smog of London in December 1952. This spurred epidemiological research in the United Kingdom, Holland and elsewhere.[221] In 1953, George L. Waldbott, an American allergist, first described a new disease he named smoker's respiratory syndrome in the 1953 Journal of the American Medical Association. This was the first association between tobacco smoking and chronic respiratory disease.[222]
Modern treatments were developed during the second half of the 20th century. Evidence supporting the use of steroids in COPD was published in the late 1950s. Bronchodilators came into use in the 1960s following a promising trial of isoprenaline. Further bronchodilators, such as short-acting salbutamol, were developed in the 1970s and the use of long-acting bronchodilators began in the mid-1990s.[223]
Society and culture
[edit]It is generally accepted that COPD is widely underdiagnosed and many people remain untreated. In the US the NIH has promoted November as COPD Awareness Month to be an annual focus on increasing awareness of the condition.[224]
Economics
[edit]Globally, as of 2010, COPD is estimated to result in economic costs of $2.1 trillion, half of which occurring in the developing world.[225] Of this total an estimated $1.9 trillion are direct costs such as medical care, while $0.2 trillion are indirect costs such as missed work.[226] This is expected to more than double by 2030.[225] In Europe, COPD represents 3% of healthcare spending.[131] In the United States, costs of the disease are estimated at $50 billion, most of which is due to exacerbation.[118] COPD was among the most expensive conditions seen in U.S. hospitals in 2011, with a total cost of about $5.7 billion.[217]
Research
[edit]Hyaluronan is a natural sugar in the extracellular matrix that provides a protective coating for cells. It has been shown that on exposure to pollution the hyaluronan in the lungs breaks down into fragments causing irritation and activation of the immune system. There follows subsequent airway constriction and inflammation. The study showed that the inhalation of unfragmented hyaluronan overcame the effects of fragmented HA and reduced inflammation. Inhaled HA only acts locally in the bronchial tree and does not interfere with any drug. It improves mucus clearance by allowing it to move more freely. Further studies are to be carried out in the US to determine optimum dosage levels.[227]
A new cryogenic treatment aimed at the chronic bronchitic subtype using a liquid nitrogen metered cryospray is being trialed and was due to complete in September 2021.[228][229]
Stem-cell therapy using mesenchymal stem cells has the potential to restore lung function and thereby improve quality of life. In June 2021 eight clinical trials had been completed and seventeen were underway. Overall, stem cell therapy has proved to be safe. The trials include the use of stem cells from different sources such as adipose tissue, bone marrow and umbilical cord blood.[230]
A procedure known as targeted lung denervation is being trialed and has been used as part of a clinical trial (2021) in a hospital in the UK. The new minimally invasive procedure which takes about an hour to carry out, places electrodes to destroy branches of the vagus nerve in the lungs. The vagus nerve is responsible for both muscle contraction and mucus secretion, which results in narrowing the airways. In those with COPD these nerves are overactive, usually as a result of smoking damage and the constant mucus secretion and airway constriction leads to the symptoms of cough, shortness of breath, wheeze and tightness of the chest.[231]
The effectiveness of alpha-1 antitrypsin augmentation treatment for people who have alpha-1 antitrypsin deficiency is unclear.[232] A later clinical trial of double-dosing has shown some improvements in slowing the breakdown of elastin and the progression of emphysema with further studies being called for.[233]
Mass spectrometry is being studied as a diagnostic tool in COPD.[234]
Research continues into the use of telehealthcare to treat people with COPD when they experience episodes of shortness of breath; treating people remotely may reduce the number of emergency-room visits and improve the person's quality of life.[235]
Evidence is growing for the effectiveness of Astaxanthin against lung disease including COPD. Astaxanthin is a potent antioxidant with anti-inflammatory properties and more trials are said to be needed into its use.[236]
American COPD patients and their caregivers consider the following COPD-related research areas as the most important:
- family/social/community research
- patients' well-being
- curative research
- biomedical therapies
- policy
- holistic therapies.[237]
Other animals
[edit]Chronic obstructive pulmonary disease may occur in a number of other animals and may be caused by exposure to tobacco smoke.[238] Most cases of the disease, however, are relatively mild.[239] In horses it is known as recurrent airway obstruction (RAO) or heaves. RAO can be quite severe and most often is linked to exposure to common allergens.[240] COPD is also commonly found in old dogs.[241]
See also
[edit]References
[edit]- ^ a b c d e f g h i "Chronic obstructive pulmonary disease". nice.org. Retrieved 5 July 2021.
- ^ a b c "Chronic obstructive pulmonary disease (COPD) — Complications". BMJ Best Practice. Retrieved 11 July 2021.
- ^ a b c d e f g "Chronic obstructive pulmonary disease (COPD)". Fact Sheets. World Health Organization. Retrieved 1 July 2021.
- ^ a b c d e f g h i j k l Gold Report 2021, pp. 20–27, Chapter 2: Diagnosis and initial assessment.
- ^ a b c d Gold Report 2021, pp. 33–35, Chapter 2: Diagnosis and initial assessment.
- ^ a b c d e f g h i j k Gold Report 2021, pp. 40–46, Chapter 3: Evidence supporting prevention and maintenance therapy.
- ^ a b GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ 2024 GOLD Report, p. 15, Chapter 1: Definition and overview.
- ^ "2024 GOLD Report". Global Initiative for Chronic Obstructive Lung Disease - GOLD. Retrieved 2024-02-23.
- ^ a b c d e Myc LA, Shim YM, Laubach VE, Dimastromatteo J (April 2019). "Role of medical and molecular imaging in COPD". Clin Transl Med. 8 (1): 12. doi:10.1186/s40169-019-0231-z. PMC 6465368. PMID 30989390.
- ^ a b "ICD-11 - ICD-11 for Mortality and Morbidity Statistics". icd.who.int. Retrieved 30 June 2021.
- ^ Martini K, Frauenfelder T (November 2020). "Advances in imaging for lung emphysema". Ann Transl Med. 8 (21): 1467. doi:10.21037/atm.2020.04.44. PMC 7723580. PMID 33313212.
- ^ a b c d e f g h i j k l m n o p q r Gold Report 2021, pp. 8–14, Chapter 1: Definition and overview.
- ^ a b c d e f g h i j k Gold Report 2021, pp. 4–8, Chapter 1: Definition and overview.
- ^ a b De Rose V, Molloy K, Gohy S, Pilette C, Greene CM (2018). "Airway Epithelium Dysfunction in Cystic Fibrosis and COPD". Mediators Inflamm. 2018: 1309746. doi:10.1155/2018/1309746. PMC 5911336. PMID 29849481.
- ^ GINA and GOLD joint guidelines Ga (2014). "Asthma COPD and asthma COPD overlap syndrome (ACOS)" (PDF). GINA Guidelines.
- ^ Agusti À, Soriano JB (January 2008). "COPD as a Systemic Disease". COPD: Journal of Chronic Obstructive Pulmonary Disease. 5 (2): 133–138. doi:10.1080/15412550801941349. ISSN 1541-2555. PMID 18415812. S2CID 32732993.
- ^ a b c d e "Chronic obstructive pulmonary disease (COPD) - Aetiology | BMJ Best Practice". bestpractice.bmj.com. Retrieved 25 November 2022.
- ^ a b "COPD causes - occupations and substances". www.hse.gov.uk. Retrieved 3 July 2021.
- ^ Torres-Duque CA, García-Rodriguez MC, González-García M (August 2016). "Is Chronic Obstructive Pulmonary Disease Caused by Wood Smoke a Different Phenotype or a Different Entity?". Archivos de Bronconeumologia. 52 (8): 425–31. doi:10.1016/j.arbres.2016.04.004. PMID 27207325.
- ^ a b c d Gold Report 2021, pp. 80–83, Chapter 4: Management of stable COPD.
- ^ a b c d e f g Gold Report 2021, pp. 60–65, Chapter 3: Evidence supporting prevention and maintenance therapy.
- ^ Dobler CC, Morrow AS, Beuschel B, Farah MH, Majzoub AM, Wilson ME, et al. (March 2020). "Pharmacologic Therapies in Patients With Exacerbation of Chronic Obstructive Pulmonary Disease: A Systematic Review With Meta-analysis". Annals of Internal Medicine. 172 (6): 413–422. doi:10.7326/M19-3007 (inactive 7 December 2024). PMID 32092762. S2CID 211476101.
{{cite journal}}: CS1 maint: DOI inactive as of December 2024 (link) - ^ Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (GBD 2015 Mortality and Causes of Death Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ GBD 2013 Mortality and Causes of Death Collaborators (January 2015). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
- ^ "The top 10 causes of death". www.who.int. Retrieved 2024-08-12.
- ^ "COPD Costs". www.cdc.gov. 5 July 2019. Archived from the original on 9 February 2020.
- ^ "COPD commissioning toolkit" (PDF). www.assets.publishing.service.gov.uk. Retrieved 18 July 2021.
- ^ a b Mayer AF, Karloh M, Dos Santos K, de Araujo CL, Gulart AA (March 2018). "Effects of acute use of pursed-lips breathing during exercise in patients with COPD: a systematic review and meta-analysis". Physiotherapy. 104 (1): 9–17. doi:10.1016/j.physio.2017.08.007. PMID 28969859.
- ^ Gold Report 2021, pp. 90–96, Chapter 4: Management of stable COPD.
- ^ O'Donnell DE, Milne KM, James MD, de Torres JP, Neder JA (January 2020). "Dyspnea in COPD: New Mechanistic Insights and Management Implications". Advances in Therapy. 37 (1): 41–60. doi:10.1007/s12325-019-01128-9. PMC 6979461. PMID 31673990.
- ^ a b Szalontai K, Gémes N, Furák J, et al. (June 2021). "Chronic Obstructive Pulmonary Disease: Epidemiology, Biomarkers, and Paving the Way to Lung Cancer". J Clin Med. 10 (13): 2889. doi:10.3390/jcm10132889. PMC 8268950. PMID 34209651.
- ^ a b c d e f g h i j Gold Report 2021, pp. 104–109, Chapter 5: Management of exacerbations.
- ^ "Common Colds". Centers for Disease Control and Prevention. 7 October 2020. Archived from the original on June 18, 2023. Retrieved 20 August 2021.
- ^ Guo-Parke H, Linden D, Weldon S, Kidney JC, Taggart CC (2020). "Mechanisms of Virus-Induced Airway Immunity Dysfunction in the Pathogenesis of COPD Disease, Progression, and Exacerbation". Frontiers in Immunology. 11: 1205. doi:10.3389/fimmu.2020.01205. PMC 7325903. PMID 32655557.
- ^ Short B, Carson S, Devlin AC, et al. (March 2021). "Non-typeable Haemophilus influenzae chronic colonization in chronic obstructive pulmonary disease (COPD)". Critical Reviews in Microbiology. 47 (2): 192–205. doi:10.1080/1040841X.2020.1863330. PMID 33455514. S2CID 230608674.
- ^ US EPA O (19 April 2016). "Particulate Matter (PM) Basics". www.epa.gov. Retrieved 21 July 2021.
- ^ a b Halpin DM, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, Agusti AA, Vogelmeier CF (January 2021). "Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease". Am J Respir Crit Care Med. 203 (1): 24–36. doi:10.1164/rccm.202009-3533SO. PMC 7781116. PMID 33146552.
- ^ Iacobucci G (August 2020). "Covid lockdown: England sees fewer cases of colds, flu, and bronchitis". BMJ. 370: m3182. doi:10.1136/bmj.m3182. PMID 32784206. S2CID 221097739.
- ^ US EPA O (13 August 2019). "Health Effects Attributed to Wildfire Smoke". www.epa.gov. Retrieved 21 July 2021.
- ^ "Forest Fires and Lung Health". the lung association. 25 August 2014. Retrieved 21 July 2021.
- ^ "Bushfire smoke". National Centre for Farmer Health. 19 March 2014. Retrieved 21 July 2021.
- ^ Luo J, Zhang D, Tang W, Dou LY, Sun Y (2021). "Impact of Frailty on the Risk of Exacerbations and All-Cause Mortality in Elderly Patients with Stable Chronic Obstructive Pulmonary Disease". Clin Interv Aging. 16: 593–601. doi:10.2147/CIA.S303852. PMC 8053481. PMID 33880018.
- ^ Aleva FE, Voets LW, Simons SO, de Mast Q, van der Ven AJ, Heijdra YF (March 2017). "Prevalence and Localization of Pulmonary Embolism in Unexplained Acute Exacerbations of COPD: A Systematic Review and Meta-analysis". Chest. 151 (3): 544–554. doi:10.1016/j.chest.2016.07.034. PMID 27522956. S2CID 7181799.
- ^ a b c d e f Gold Report 2021, pp. 26–33, Chapter 2: Diagnosis and initial assessment.
- ^ Edgar RG, Patel M, Bayliss S, Crossley D, Sapey E, Turner AM (2017). "Treatment of lung disease in alpha-1 antitrypsin deficiency: a systematic review". Int J Chron Obstruct Pulmon Dis. 12: 1295–1308. doi:10.2147/COPD.S130440. PMC 5422329. PMID 28496314.
- ^ Gold Report 2021, pp. 26–33, 2. Diagnosis and initial assessment.
- ^ a b c d Clementi EA, Talusan A, Vaidyanathan S, Veerappan A, Mikhail M, Ostrofsky D, et al. (January 2019). "Metabolic Syndrome and Air Pollution: A Narrative Review of Their Cardiopulmonary Effects". Toxics. 7 (1): 6. doi:10.3390/toxics7010006. PMC 6468691. PMID 30704059.
- ^ Chan SH, Selemidis S, Bozinovski S, Vlahos R (June 2019). "Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): clinical significance and therapeutic strategies". Pharmacol Ther. 198: 160–188. doi:10.1016/j.pharmthera.2019.02.013. PMC 7112632. PMID 30822464.
- ^ a b Forfia PR, Vaidya A, Wiegers SE (January 2013). "Pulmonary heart disease: The heart-lung interaction and its impact on patient phenotypes". Pulm Circ. 3 (1): 5–19. doi:10.4103/2045-8932.109910. PMC 3641739. PMID 23662171.
- ^ a b c Olsson KM, Corte TJ, Kamp JC, Montani D, Nathan SD, Neubert L, Price LC, Kiely DG (September 2023). "Pulmonary hypertension associated with lung disease: new insights into pathomechanisms, diagnosis, and management". The Lancet. Respiratory Medicine. 11 (9): 820–835. doi:10.1016/S2213-2600(23)00259-X. PMID 37591300.
- ^ Gold Report 2021, pp. 121–126, Chapter 6: COPD and comorbidities.
- ^ Singh D, Mathioudakis AG, Higham A (March 2022). "Chronic obstructive pulmonary disease and COVID-19: interrelationships". Curr Opin Pulm Med. 28 (2): 76–83. doi:10.1097/MCP.0000000000000834. PMC 8815646. PMID 34690257.
- ^ a b Weinberger SE (2019). "6. Chronic Obstructive Pulmonary Disease". Principles of pulmonary medicine (7th ed.). Elsevier. p. 104. doi:10.1016/B978-0-323-52371-4.00009-X. ISBN 978-0-323-52371-4.
- ^ Delaunois L (October 1989). "Anatomy and physiology of collateral respiratory pathways". The European Respiratory Journal. 2 (9): 893–904. doi:10.1183/09031936.93.02090893. PMID 2680588. S2CID 7124561. Retrieved 30 August 2021.
- ^ Ramírez-Venegas A, Torres-Duque CA, Guzmán-Bouilloud NE, González-García M, Sansores RH (2019). "SMALLa AIRWAY DISEASE IN COPD ASSOCIATED TO BIOMASS EXPOSURE". Rev Invest Clin. 71 (1): 70–78. doi:10.24875/RIC.18002652. PMID 30810542.
- ^ Corlateanu A, Mendez Y, Wang Y, Garnica RJ, Botnaru V, Siafakas N (2020). "Chronic obstructive pulmonary disease and phenotypes: a state-of-the-art". Pulmonology. 26 (2): 95–100. doi:10.1016/j.pulmoe.2019.10.006. PMID 31740261.
- ^ Postma DS, Rabe KF (24 September 2015). "The Asthma–COPD Overlap Syndrome". New England Journal of Medicine. 373 (13): 1241–1249. doi:10.1056/NEJMra1411863. PMID 26398072.
- ^ Mekov E, Nuñez A, Sin DD, Ichinose M, Rhee CK, Maselli DJ, Coté A, Suppli Ulrik C, Maltais F, Anzueto A, Miravitlles M (June 2021). "Update on Asthma–COPD Overlap (ACO): A Narrative Review". International Journal of Chronic Obstructive Pulmonary Disease. 16: 1783–1799. doi:10.2147/COPD.S312560. PMC 8216660. PMID 34168440.
- ^ a b Halpin DM, Miravitlles M, Metzdorf N, Celli B (2017). "Impact and prevention of severe exacerbations of COPD: a review of the evidence". Int J Chron Obstruct Pulmon Dis. 12: 2891–2908. doi:10.2147/COPD.S139470. PMC 5638577. PMID 29062228.
- ^ a b Brightling C, Greening N (August 2019). "Airway inflammation in COPD: progress to precision medicine" (PDF). Eur Respir J. 54 (2). doi:10.1183/13993003.00651-2019. PMID 31073084. S2CID 149444134.
- ^ a b c d e Gold Report 2021, pp. 54–58, Chapter 3: Evidence supporting prevention and maintenance therapy.
- ^ Bai S, Zhao L (2021). "Imbalance Between Injury and Defense in the COPD Emphysematous Phenotype". Frontiers in Medicine. 8: 653332. doi:10.3389/fmed.2021.653332. PMC 8131650. PMID 34026786.
- ^ Kumar A, Mahajan A, Salazar EA, et al. (June 2021). "Impact of human immunodeficiency virus on pulmonary vascular disease". Glob Cardiol Sci Pract. 2021 (2): e202112. doi:10.21542/gcsp.2021.12. PMC 8272407. PMID 34285903.
- ^ Osadnik CR, McDonald CF, Holland AE (2014). "Clinical issues of mucus accumulation in COPD". Int J Chron Obstruct Pulmon Dis. 9: 301–2. doi:10.2147/COPD.S61797. PMC 3970915. PMID 24741301.
- ^ a b Silverman EK (February 2020). "Genetics of COPD". Annu Rev Physiol. 82: 413–431. doi:10.1146/annurev-physiol-021317-121224. PMC 7193187. PMID 31730394.
- ^ "FAQs Dust - Health and Safety Executive (HSE)".
- ^ a b Nikolić MZ, Sun D, Rawlins EL (15 August 2018). "Human lung development: recent progress and new challenges". Development. 145 (16). doi:10.1242/dev.163485. PMC 6124546. PMID 30111617.
- ^ Smith BM, Traboulsi H, Austin JM, et al. (January 2018). "Human airway branch variation and chronic obstructive pulmonary disease". Proc Natl Acad Sci U S A. 115 (5): E974–E981. Bibcode:2018PNAS..115E.974S. doi:10.1073/pnas.1715564115. PMC 5798356. PMID 29339516.
- ^ a b Bailey KL, Samuelson DR, Wyatt TA (February 2021). "Alcohol use disorder: A pre-existing condition for COVID-19?". Alcohol. 90: 11–17. doi:10.1016/j.alcohol.2020.10.003. PMC 7568767. PMID 33080339.
- ^ a b Smith P, Jeffers LA, Koval M (November 2019). "Effects of different routes of endotoxin injury on barrier function in alcoholic lung syndrome". Alcohol. 80: 81–89. doi:10.1016/j.alcohol.2018.08.007. PMC 6613986. PMID 31278041.
- ^ Arvers P (December 2018). "[Alcohol consumption and lung damage: Dangerous relationships]". Rev Mal Respir (in French). 35 (10): 1039–1049. doi:10.1016/j.rmr.2018.02.009. PMID 29941207. S2CID 239523761.
- ^ Slovinsky WS, Romero F, Sales D, Shaghaghi H, Summer R (November 2019). "The involvement of GM-CSF deficiencies in parallel pathways of pulmonary alveolar proteinosis and the alcoholic lung". Alcohol. 80: 73–79. doi:10.1016/j.alcohol.2018.07.006. PMC 6592783. PMID 31229291.
- ^ a b Ward H (2012). Oxford Handbook of Epidemiology for Clinicians. Oxford University Press. pp. 289–290. ISBN 978-0-19-165478-7.
- ^ Rennard S (2013). Clinical management of chronic obstructive pulmonary disease (2nd ed.). Informa Healthcare. p. 23. ISBN 978-0-8493-7588-0.
- ^ Sharma A, Barclay J (2010). COPD in primary care. Radcliffe Pub. p. 9. ISBN 978-1-84619-316-3.
- ^ Han MK, Martinez FJ (September 2020). "Host, Gender, and Early-Life Factors as Risks for Chronic Obstructive Pulmonary Disease". Clinics in Chest Medicine. 41 (3): 329–337. doi:10.1016/j.ccm.2020.06.009. PMC 7993923. PMID 32800188.
- ^ Amaral AF, Strachan DP, Burney PG, Jarvis DL (May 2017). "Female Smokers Are at Greater Risk of Airflow Obstruction Than Male Smokers. UK Biobank" (PDF). American Journal of Respiratory and Critical Care Medicine. 195 (9): 1226–1235. doi:10.1164/rccm.201608-1545OC. hdl:10044/1/45106. PMID 28075609. S2CID 9360093.
- ^ "Tobacco". Fact Sheets. World Health Organization. Retrieved 12 July 2021.
- ^ Savran O, Ulrik CS (2018). "Early life insults as determinants of chronic obstructive pulmonary disease in adult life". International Journal of Chronic Obstructive Pulmonary Disease. 13: 683–693. doi:10.2147/COPD.S153555. PMC 5834168. PMID 29520136.
- ^ Patel MP, Khangoora VS, Marik PE (October 2019). "A Review of the Pulmonary and Health Impacts of Hookah Use". Annals of the American Thoracic Society. 16 (10): 1215–1219. doi:10.1513/AnnalsATS.201902-129CME. PMID 31091965. S2CID 155103502.
- ^ a b c d Chatkin JM, Zabert G, Zabert I, Chatkin G, Jiménez-Ruiz CA, de Granda-Orive JI, et al. (September 2017). "Lung Disease Associated With Marijuana Use". Arch Bronconeumol. 53 (9): 510–515. doi:10.1016/j.arbres.2017.03.019. PMID 28483343.
- ^ Underner M, Urban T, Perriot J, Peiffer G, Harika-Germaneau G, Jaafari N (December 2018). "[Spontaneous pneumothorax and lung emphysema in cannabis users]". Rev Pneumol Clin (in French). 74 (6): 400–415. doi:10.1016/j.pneumo.2018.06.003. PMID 30420278. S2CID 59233744.
- ^ Martinasek MP, McGrogan JB, Maysonet A (November 2016). "A Systematic Review of the Respiratory Effects of Inhalational Marijuana". Respir Care. 61 (11): 1543–1551. doi:10.4187/respcare.04846. PMID 27507173.
- ^ Ribeiro LI, Ind PW (October 2016). "Effect of cannabis smoking on lung function and respiratory symptoms: a structured literature review". npj Primary Care Respiratory Medicine. 26: 16071. doi:10.1038/npjpcrm.2016.71. PMC 5072387. PMID 27763599.
- ^ "Access to clean fuels and technologies for cooking". Our World in Data. Retrieved 15 February 2020.
- ^ Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM (September 2006). "Global burden of COPD: systematic review and meta-analysis". The European Respiratory Journal. 28 (3): 523–32. doi:10.1183/09031936.06.00124605. PMID 16611654.
- ^ a b c d e f g h Pirozzi C, Scholand MB (July 2012). "Smoking cessation and environmental hygiene". The Medical Clinics of North America. 96 (4): 849–67. doi:10.1016/j.mcna.2012.04.014. PMID 22793948.
- ^ Hopper T (2014). Mosby's Pharmacy Technician – E-Book: Principles and Practice. Elsevier Health Sciences. p. 610. ISBN 978-0-323-29245-0.
- ^ Barnes PJ, Drazen JM, Rennard SI (2009). "Relationship between cigarette smoking and occupational exposures". In Barnes PJ, Drazen JM, Rennard SI, Thomson NC (eds.). Asthma and COPD: Basic Mechanisms and Clinical Management. Academic. p. 464. ISBN 978-0-12-374001-4.
- ^ a b Foreman MG, Campos M, Celedón JC (July 2012). "Genes and chronic obstructive pulmonary disease". The Medical Clinics of North America. 96 (4): 699–711. doi:10.1016/j.mcna.2012.02.006. PMC 3399759. PMID 22793939.
- ^ Brode SK, Ling SC, Chapman KR (September 2012). "Alpha-1 antitrypsin deficiency: a commonly overlooked cause of lung disease". CMAJ. 184 (12): 1365–71. doi:10.1503/cmaj.111749. PMC 3447047. PMID 22761482.
- ^ a b c d e Reilly JJ, Silverman EK, Shapiro SD (2011). "Chronic Obstructive Pulmonary Disease". In Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J (eds.). Harrison's Principles of Internal Medicine (18th ed.). McGraw Hill. pp. 2151–9. ISBN 978-0-07-174889-6.
- ^ "Home - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 17 July 2022.
- ^ Maselli DJ, Bhatt SP, Anzueto A, Bowler RP, DeMeo DL, Diaz AA, et al. (August 2019). "Clinical Epidemiology of COPD: Insights From 10 Years of the COPDGene Study". Chest. 156 (2): 228–238. doi:10.1016/j.chest.2019.04.135. PMC 7198872. PMID 31154041.
- ^ Capron T, Bourdin A, Perez T, Chanez P (June 2019). "COPD beyond proximal bronchial obstruction: phenotyping and related tools at the bedside" (PDF). Eur Respir Rev. 28 (152): 190010. doi:10.1183/16000617.0010-2019. PMC 9488991. PMID 31285287. S2CID 195844108.
- ^ Kumar V, Abbas AK, Aster JC (2018). Robbins basic pathology (10th ed.). Elsevier. pp. 498–502. ISBN 978-0-323-35317-5.
- ^ a b D'Ascanio M, Viccaro F, Calabrò N, Guerrieri G, Salvucci C, Pizzirusso D, et al. (2020). "Assessing Static Lung Hyperinflation by Whole-Body Plethysmography, Helium Dilution, and Impulse Oscillometry System (IOS) in Patients with COPD". Int J Chron Obstruct Pulmon Dis. 15: 2583–9. doi:10.2147/COPD.S264261. PMC 7585810. PMID 33116475.
- ^ Knox-Brown B, Patel J, Potts J, Ahmed R, Aquart-Stewart A, Cherkaski H, et al. (January 2023). "Small airways obstruction and its risk factors in the Burden of Obstructive Lung Disease (BOLD) study: a multinational cross-sectional study". Lancet Glob Health. 11 (1): e69–e82. doi:10.1016/S2214-109X(22)00456-9. hdl:10044/1/100800. PMID 36521955. S2CID 254665705.
- ^ Knox-Brown B, Patel J, Potts J, et al. (23 May 2023). "The association of spirometric small airways obstruction with respiratory symptoms, cardiometabolic diseases, and quality of life: results from the Burden of Obstructive Lung Disease (BOLD) study". Respiratory Research. 24 (1): 137. doi:10.1186/s12931-023-02450-1. PMC 10207810. PMID 37221593.
- ^ Knox-Brown B, Potts J, Santofimio VQ, Minelli C, Patel J, Abass NM, Agarwal D, Ahmed R, Mahesh PA, Bs J, Denguezli M, Franssen F, Gislason T, Janson C, Juvekar SK, Koul P, Malinovschi A, Nafees AA, Nielsen R, Paraguas SN, Buist S, Burney PG, Amaral AF (November 2023). "Isolated small airways obstruction predicts future chronic airflow obstruction: a multinational longitudinal study". BMJ Open Respiratory Research. 10 (1): e002056. doi:10.1136/bmjresp-2023-002056. hdl:20.500.11815/4593. PMC 10660204. PMID 37989490.
- ^ Lo Bello F, Ieni A, Hansbro PM, Ruggeri P, Di Stefano A, Nucera F, et al. (May 2020). "Role of the mucins in pathogenesis of COPD: implications for therapy". Expert Review of Respiratory Medicine. 14 (5): 465–483. doi:10.1080/17476348.2020.1739525. hdl:10453/139160. PMID 32133884. S2CID 212416323.
- ^ a b Higham A, Quinn AM, Cançado JE, Singh D (March 2019). "The pathology of small airways disease in COPD: historical aspects and future directions". Respir Res. 20 (1): 49. doi:10.1186/s12931-019-1017-y. PMC 6399904. PMID 30832670.
- ^ a b Calverley PM, Koulouris NG (January 2005). "Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology". The European Respiratory Journal. 25 (1): 186–99. doi:10.1183/09031936.04.00113204. PMID 15640341.
- ^ Currie GP (2010). ABC of COPD (2nd ed.). Wiley-Blackwell, BMJ Books. p. 32. ISBN 978-1-4443-2948-3.
- ^ O'Donnell DE (April 2006). "Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease". Proceedings of the American Thoracic Society. 3 (2): 180–4. doi:10.1513/pats.200508-093DO. PMID 16565429. S2CID 20644418.
- ^ Cooper CB (October 2006). "The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function". The American Journal of Medicine. 119 (10 Suppl 1): 21–31. doi:10.1016/j.amjmed.2006.08.004. PMID 16996896.
- ^ a b c "Chronic obstructive pulmonary disease - NICE Pathways". pathways.nice.org.uk. 9 April 2020. Retrieved 29 June 2021.
- ^ a b c d Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. (August 2011). "Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society". Annals of Internal Medicine. 155 (3): 179–191. doi:10.7326/0003-4819-155-3-201108020-00008. PMID 21810710. S2CID 18830625.
- ^ a b Young VB (2010). Blueprints medicine (5th ed.). Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 69. ISBN 978-0-7817-8870-0.
- ^ a b c National Institute for Health and Clinical Excellence. Clinical guideline 101: Chronic Obstructive Pulmonary Disease. London, June 2010.
- ^ Bailey KL (July 2012). "The importance of the assessment of pulmonary function in COPD". The Medical Clinics of North America. 96 (4): 745–752. doi:10.1016/j.mcna.2012.04.011. PMC 3998207. PMID 22793942.
- ^ Williams N (August 2017). "The MRC breathlessness scale". Occup Med (Lond). 67 (6): 496–497. doi:10.1093/occmed/kqx086. PMID 28898975.
- ^ a b "Chronic obstructive pulmonary disease (COPD) - Criteria | BMJ Best Practice". bestpractice.bmj.com. Retrieved 2 July 2021.
- ^ "COPD Assessment Test (CAT)". American Thoracic Society. Archived from the original on December 3, 2013. Retrieved November 29, 2013.
- ^ Tyagi J, Moola S, Bhaumik S (June 2021). "Diagnostic accuracy of screening tools for chronic obstructive pulmonary disease in primary health care: Rapid evidence synthesis". Journal of Family Medicine and Primary Care. 10 (6): 2184–2194. doi:10.4103/jfmpc.jfmpc_2263_20. PMC 8284240. PMID 34322411.
- ^ "saber sheath trachea | Search | Radiopaedia.org". Radiopaedia. Retrieved 13 August 2021.
- ^ a b Vestbo J, Hurd SS, Agustí AG, et al. (February 2013). "Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary". American Journal of Respiratory and Critical Care Medicine. 187 (4): 350–352. doi:10.1164/rccm.201204-0596PP. PMID 22878278.
- ^ BTS COPD Consortium (2005). "Spirometry in practice – a practical guide to using spirometry in primary care". pp. 8–9. Archived from the original on 26 August 2014. Retrieved 25 August 2014.
- ^ a b c Policy Recommendations for Smoking Cessation and Treatment of Tobacco Dependence. World Health Organization. 2003. pp. 15–40. ISBN 978-92-4-156240-9. Archived from the original on 2008-09-15.
- ^ Jiménez-Ruiz CA, Fagerström KO (March 2013). "Smoking cessation treatment for COPD smokers: the role of counselling". Monaldi Archives for Chest Disease. 79 (1): 33–7. doi:10.4081/monaldi.2013.107. PMID 23741944.
- ^ a b "Chronic obstructive pulmonary disease in over 16s: diagnosis and management | Guidance and guidelines | NICE". www.nice.org.uk. 23 June 2010. Retrieved 2018-06-05.
- ^ Kumar P, Clark M (2005). Clinical Medicine (6th ed.). Elsevier Saunders. pp. 900–1. ISBN 978-0-7020-2763-5.
- ^ a b Tønnesen P (March 2013). "Smoking cessation and COPD". European Respiratory Review. 22 (127): 37–43. doi:10.1183/09059180.00007212. PMC 9487432. PMID 23457163.
- ^ "Coping with cravings". nhs.uk. 27 April 2018. Retrieved 15 July 2021.
- ^ van Eerd EA, van der Meer RM, van Schayck OC, Kotz D (August 2016). "Smoking cessation for people with chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2019 (8): CD010744. doi:10.1002/14651858.CD010744.pub2. PMC 6400424. PMID 27545342.
- ^ Smith BK, Timby NE (2005). Essentials of nursing : care of adults and children. Lippincott Williams & Wilkins. p. 338. ISBN 978-0-7817-5098-1.
- ^ Rom WN, Markowitz SB, eds. (2007). Environmental and occupational medicine (4th ed.). Wolters Kluwer/Lippincott Williams & Wilkins. pp. 521–522. ISBN 978-0-7817-6299-1.
- ^ "Wet cutting". Health and Safety Executive. Archived from the original on December 3, 2013. Retrieved November 29, 2013.
- ^ George RB (2005). Chest medicine : essentials of pulmonary and critical care medicine (5th ed.). Lippincott Williams & Wilkins. p. 172. ISBN 978-0-7817-5273-2.
- ^ a b c d e f g h i j Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. (February 2013). "Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary". American Journal of Respiratory and Critical Care Medicine. 187 (4): 347–365. doi:10.1164/rccm.201204-0596PP. PMID 22878278.
- ^ "Chronic obstructive pulmonary disease (COPD) - Treatment - NHS". 20 October 2017.
- ^ CDC (2024-01-18). "RSV information for healthcare providers". Centers for Disease Control and Prevention. Retrieved 2024-02-23.
- ^ "2024 GOLD Report". Global Initiative for Chronic Obstructive Lung Disease - GOLD. Retrieved 2024-02-23.
- ^ Howcroft M, Walters EH, Wood-Baker R, Walters JA (December 2016). "Action plans with brief patient education for exacerbations in chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2016 (12): CD005074. doi:10.1002/14651858.CD005074.pub4. PMC 6463844. PMID 27990628.
- ^ Lenferink A, Brusse-Keizer M, van der Valk PD, Frith PA, Zwerink M, Monninkhof EM, et al. (August 2017). "Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2017 (8): CD011682. doi:10.1002/14651858.CD011682.pub2. PMC 6483374. PMID 28777450.
- ^ Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, et al. (April 2019). "Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials". Thorax. 74 (4): 337–345. doi:10.1136/thoraxjnl-2018-212092. PMID 30630893.
- ^ a b Vollenweider DJ, Frei A, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA (October 2018). "Antibiotics for exacerbations of chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2018 (10): CD010257. doi:10.1002/14651858.CD010257.pub2. PMC 6517133. PMID 30371937.
- ^ Mackay AJ, Hurst JR (July 2012). "COPD exacerbations: causes, prevention, and treatment". The Medical Clinics of North America. 96 (4): 789–809. doi:10.1016/j.mcna.2012.02.008. PMID 22793945.
- ^ "Fluoroquinolone Antibacterial Drugs: Drug Safety Communication – FDA Advises Restricting Use for Certain Uncomplicated Infections". FDA. 12 May 2016. Archived from the original on 16 May 2016. Retrieved 16 May 2016.
- ^ a b c d e f g h Decramer M, Janssens W, Miravitlles M (April 2012). "Chronic obstructive pulmonary disease". Lancet. 379 (9823): 1341–51. CiteSeerX 10.1.1.1000.1967. doi:10.1016/S0140-6736(11)60968-9. PMC 7172377. PMID 22314182.
- ^ Oliveira EP, Medeiros Junior P (2020). "Palliative care in pulmonary medicine". Jornal Brasileiro de Pneumologia (in Portuguese). 46 (3): e20190280. doi:10.36416/1806-3756/e20190280. PMC 7572288. PMID 32638839.
- ^ Wilson ME, Dobler CC, Morrow AS, Beuschel B, Alsawas M, Benkhadra R, et al. (February 2020). "Association of Home Noninvasive Positive Pressure Ventilation With Clinical Outcomes in Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-analysis". JAMA. 323 (5): 455–465. doi:10.1001/jama.2019.22343. PMC 7042860. PMID 32016309.
- ^ Almadhoun K, Sharma S (2021). "Bronchodilators". StatPearls. StatPearls Publishing. PMID 30085570. Retrieved 27 July 2021.
- ^ a b c Gold Report 2021, pp. 48–52, Chapter 3: Evidence supporting prevention and maintenance therapy.
- ^ Farne HA, Cates CJ (October 2015). "Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease" (PDF). The Cochrane Database of Systematic Reviews. 2015 (10): CD008989. doi:10.1002/14651858.CD008989.pub3. PMC 4164463. PMID 26490945.
- ^ Oba Y, Keeney E, Ghatehorde N, Dias S, et al. (Cochrane Airways Group) (December 2018). "Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis". The Cochrane Database of Systematic Reviews. 2018 (12): CD012620. doi:10.1002/14651858.CD012620.pub2. PMC 6517098. PMID 30521694.
- ^ a b "Recommendations | Chronic obstructive pulmonary disease in over 16s: diagnosis and management | Guidance | NICE". www.nice.org.uk. 5 December 2018. Retrieved 1 August 2021.
- ^ a b Cave AC, Hurst MM (May 2011). "The use of long acting β₂-agonists, alone or in combination with inhaled corticosteroids, in chronic obstructive pulmonary disease (COPD): a risk-benefit analysis". Pharmacology & Therapeutics. 130 (2): 114–143. doi:10.1016/j.pharmthera.2010.12.008. PMID 21276815.
- ^ Spencer S, Karner C, Cates CJ, Evans DJ (December 2011). Spencer S (ed.). "Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2011 (12): CD007033. doi:10.1002/14651858.CD007033.pub3. PMC 6494276. PMID 22161409.
- ^ Wang J, Nie B, Xiong W, Xu Y (April 2012). "Effect of long-acting beta-agonists on the frequency of COPD exacerbations: a meta-analysis". Journal of Clinical Pharmacy and Therapeutics. 37 (2): 204–211. doi:10.1111/j.1365-2710.2011.01285.x. PMID 21740451. S2CID 45383688.
- ^ a b Geake JB, Dabscheck EJ, Wood-Baker R, Cates CJ (January 2015). "Indacaterol, a once-daily beta2-agonist, versus twice-daily beta₂-agonists or placebo for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 1 (3): CD010139. doi:10.1002/14651858.CD010139.pub2. PMC 6464646. PMID 25575340.
- ^ Decramer ML, Hanania NA, Lötvall JO, Yawn BP (2013). "The safety of long-acting β2-agonists in the treatment of stable chronic obstructive pulmonary disease". International Journal of Chronic Obstructive Pulmonary Disease. 8: 53–64. doi:10.2147/COPD.S39018. PMC 3558319. PMID 23378756.
- ^ Nannini LJ, Lasserson TJ, Poole P (September 2012). Nannini LJ (ed.). "Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 9 (9): CD006829. doi:10.1002/14651858.CD006829.pub2. PMC 4170910. PMID 22972099.
- ^ Fukuda N, Horita N, Kaneko A, Goto A, Kaneko T, Ota E, Kew KM (2023-06-05). "Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2023 (6): CD012066. doi:10.1002/14651858.CD012066.pub3. ISSN 1469-493X. PMC 10241721. PMID 37276335.
- ^ Zheng Y, Zhu J, Liu Y, Lai W, Lin C, Qiu K, et al. (November 2018). "Triple therapy in the management of chronic obstructive pulmonary disease: systematic review and meta-analysis". BMJ. 363: k4388. doi:10.1136/bmj.k4388. PMC 6218838. PMID 30401700.
- ^ a b Karner C, Chong J, Poole P (July 2014). "Tiotropium versus placebo for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2016 (7): CD009285. doi:10.1002/14651858.CD009285.pub3. PMC 8934583. PMID 25046211.
- ^ Cheyne L, Irvin-Sellers MJ, White J (September 2015). "Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2015 (9): CD009552. doi:10.1002/14651858.CD009552.pub3. PMC 8749963. PMID 26391969.
- ^ Singh S, Loke YK, Enright P, Furberg CD (January 2013). "Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications". Thorax. 68 (1): 114–6. doi:10.1136/thoraxjnl-2011-201275. PMID 22764216.
- ^ Jones P (April 2013). "Aclidinium bromide twice daily for the treatment of chronic obstructive pulmonary disease: a review". Advances in Therapy. 30 (4): 354–68. doi:10.1007/s12325-013-0019-2. PMID 23553509. S2CID 3530290.
- ^ Cazzola M, Page CP, Matera MG (June 2013). "Aclidinium bromide for the treatment of chronic obstructive pulmonary disease". Expert Opinion on Pharmacotherapy. 14 (9): 1205–14. doi:10.1517/14656566.2013.789021. PMID 23566013. S2CID 24973904.
- ^ a b Ni H, Soe Z, Moe S (September 2014). "Aclidinium bromide for stable chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 9 (9): CD010509. doi:10.1002/14651858.CD010509.pub2. PMC 8922974. PMID 25234126.
- ^ Ni H, Htet A, Moe S (June 2017). "Umeclidinium bromide versus placebo for people with chronic obstructive pulmonary disease (COPD)". The Cochrane Database of Systematic Reviews. 2017 (6): CD011897. doi:10.1002/14651858.CD011897.pub2. PMC 6481854. PMID 28631387.
- ^ Ismaila AS, Huisman EL, Punekar YS, Karabis A (2015). "Comparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysis". International Journal of Chronic Obstructive Pulmonary Disease. 10: 2495–517. doi:10.2147/COPD.S92412. PMC 4655912. PMID 26604738.
- ^ a b Chen H, Sun J, Huang Q, Liu Y, Yuan M, Ma C, et al. (2021). "Inhaled Corticosteroids and the Pneumonia Risk in Patients With Chronic Obstructive Pulmonary Disease: A Meta-analysis of Randomized Controlled Trials". Front Pharmacol. 12: 691621. doi:10.3389/fphar.2021.691621. PMC 8275837. PMID 34267661.
- ^ Chinet T, Dumoulin J, Honore I, Braun JM, Couderc LJ, Febvre M, et al. (December 2016). "[The place of inhaled corticosteroids in COPD]". Revue des Maladies Respiratoires. 33 (10): 877–891. doi:10.1016/j.rmr.2015.11.009. PMID 26831345.
- ^ Walters JA, Tan DJ, White CJ, Gibson PG, Wood-Baker R, Walters EH (September 2014). "Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2014 (9): CD001288. doi:10.1002/14651858.CD001288.pub4. PMC 11195634. PMID 25178099.
- ^ Walters JA, Tan DJ, White CJ, Wood-Baker R (March 2018). "Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2018 (3): CD006897. doi:10.1002/14651858.CD006897.pub4. PMC 6494402. PMID 29553157.
- ^ van Geffen WH, Tan DJ, Walters JA, Walters EH (2023-12-06). Cochrane Airways Group (ed.). "Inhaled corticosteroids with combination inhaled long-acting beta2-agonists and long-acting muscarinic antagonists for chronic obstructive pulmonary disease". Cochrane Database of Systematic Reviews. 2023 (12): CD011600. doi:10.1002/14651858.CD011600.pub3. PMC 10698842. PMID 38054551.
- ^ Mammen MJ, Sethi S (2012). "Macrolide therapy for the prevention of acute exacerbations in chronic obstructive pulmonary disease". Polskie Archiwum Medycyny Wewnetrznej. 122 (1–2): 54–9. doi:10.20452/pamw.1134. PMID 22353707. S2CID 35183033.
- ^ a b Herath SC, Normansell R, Maisey S, Poole P (October 2018). "Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD)". The Cochrane Database of Systematic Reviews. 2018 (10): CD009764. doi:10.1002/14651858.CD009764.pub3. PMC 6517028. PMID 30376188.
- ^ Simoens S, Laekeman G, Decramer M (May 2013). "Preventing COPD exacerbations with macrolides: a review and budget impact analysis". Respiratory Medicine. 107 (5): 637–48. doi:10.1016/j.rmed.2012.12.019. PMID 23352223.
- ^ Poole P, Sathananthan K, Fortescue R (May 2019). "Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 5 (3): CD001287. doi:10.1002/14651858.CD001287.pub6. PMC 6527426. PMID 31107966.
- ^ "Erdosteine". NICE. Retrieved 20 July 2021.
- ^ Meldrum OW, Chotirmall SH (June 2021). "Mucus, Microbiomes and Pulmonary Disease". Biomedicines. 9 (6): 675. doi:10.3390/biomedicines9060675. PMC 8232003. PMID 34199312.
- ^ "Bronchitis". nhs.uk. 17 October 2017.
- ^ Zhu A, Teng Y, Ge D, Zhang X, Hu M, Yao X (October 2019). "Role of metformin in treatment of patients with chronic obstructive pulmonary disease: a systematic review". Journal of Thoracic Disease. 11 (10): 4371–4378. doi:10.21037/jtd.2019.09.84. PMC 6837976. PMID 31737323.
- ^ a b Khor YH, Renzoni EA, Visca D, McDonald CF, Goh NS (July 2019). "Oxygen therapy in COPD and interstitial lung disease: navigating the knowns and unknowns". ERJ Open Res. 5 (3). doi:10.1183/23120541.00118-2019. PMC 6745413. PMID 31544111.
- ^ Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D (November 2016). "Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy". The Cochrane Database of Systematic Reviews. 11 (8): CD006429. doi:10.1002/14651858.CD006429.pub3. PMC 6464154. PMID 27886372.
- ^ Jindal SK (2013). Chronic Obstructive Pulmonary Disease. Jaypee Brothers Medical. p. 139. ISBN 978-93-5090-353-7.
- ^ a b O'Driscoll BR, Howard LS, Davison AG (October 2008). "BTS guideline for emergency oxygen use in adult patients". Thorax. 63 (6): vi1-68. doi:10.1136/thx.2008.102947. PMID 18838559.
- ^ "2024 GOLD Report". Global Initiative for Chronic Obstructive Lung Disease - GOLD. Retrieved 2024-02-23.
- ^ Venkatesan P (January 2024). "GOLD COPD report: 2024 update". The Lancet Respiratory Medicine. 12 (1): 15–16. doi:10.1016/S2213-2600(23)00461-7. PMID 38061380. S2CID 265751322.
- ^ "Pulmonary Rehabilitation". medlineplus.gov. Retrieved 9 September 2021.
- ^ McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA (December 2013). "Water-based exercise training for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (12): CD008290. doi:10.1002/14651858.CD008290.pub2. PMID 24353107.
- ^ Menadue C, Piper AJ, van 't Hul AJ, Wong KK (May 2014). "Non-invasive ventilation during exercise training for people with chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2014 (5): CD007714. doi:10.1002/14651858.CD007714.pub2. PMC 10984247. PMID 24823712.
- ^ a b McKeough ZJ, Velloso M, Lima VP, Alison JA (November 2016). "Upper limb exercise training for COPD". The Cochrane Database of Systematic Reviews. 2016 (11): CD011434. doi:10.1002/14651858.CD011434.pub2. PMC 6464968. PMID 27846347.
- ^ a b Ngai SP, Jones AY, Tam WW (June 2016). "Tai Chi for chronic obstructive pulmonary disease (COPD)". The Cochrane Database of Systematic Reviews. 2016 (6): CD009953. doi:10.1002/14651858.CD009953.pub2. PMC 8504989. PMID 27272131.
- ^ Ammous O, Feki W, Lotfi T, Khamis AM, Gosselink R, Rebai A, Kammoun S (2023-01-06). Cochrane Airways Group (ed.). "Inspiratory muscle training, with or without concomitant pulmonary rehabilitation, for chronic obstructive pulmonary disease (COPD)". Cochrane Database of Systematic Reviews. 2023 (1): CD013778. doi:10.1002/14651858.CD013778.pub2. PMC 9817429. PMID 36606682.
- ^ Thomas MJ, Simpson J, Riley R, Grant E (June 2010). "The impact of home-based physiotherapy interventions on breathlessness during activities of daily living in severe COPD: a systematic review". Physiotherapy. 96 (2): 108–19. doi:10.1016/j.physio.2009.09.006. PMID 20420957.
- ^ a b Wearing J, Beaumont S, Forbes D, Brown B, Engel R (February 2016). "The Use of Spinal Manipulative Therapy in the Management of Chronic Obstructive Pulmonary Disease: A Systematic Review". Journal of Alternative and Complementary Medicine. 22 (2): 108–14. doi:10.1089/acm.2015.0199. PMC 4761829. PMID 26700633.
- ^ Simonelli C, Vitacca M, Vignoni M, Ambrosino N, Paneroni M (2019). "Effectiveness of manual therapy in COPD: A systematic review of randomised controlled trials". Pulmonology. 25 (4): 236–247. doi:10.1016/j.pulmoe.2018.12.008. PMID 30738792.
- ^ a b Osadnik CR, McDonald CF, Jones AP, Holland AE (March 2012). "Airway clearance techniques for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2012 (3): CD008328. doi:10.1002/14651858.CD008328.pub2. PMC 11285303. PMID 22419331.
- ^ Ferreira IM, Brooks D, White J, Goldstein R (December 2012). Ferreira IM (ed.). "Nutritional supplementation for stable chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 12: CD000998. doi:10.1002/14651858.CD000998.pub3. PMID 23235577.
- ^ Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HA (29 August 2016). "Bronchodilators delivered by nebuliser versus inhalers for lung attacks of chronic obstructive pulmonary disease". Cochrane Database of Systematic Reviews. 29 (8): 011826. doi:10.1002/14651858.CD011826.pub2. hdl:11370/95fc3e6e-ebd0-440f-9721-489729f80add. PMC 8487315. PMID 27569680.
- ^ Wise R. "Chronic Obstructive Pulmonary Disease (COPD)". Pulmonary Disorders: Merck Manuals Professional Edition. Archived from the original on 28 December 2016. Retrieved 16 December 2016.
- ^ Osman LM, Ayres JG, Garden C, Reglitz K, Lyon J, Douglas JG (2008). "Home warmth and health status of COPD patients". Eur. J. Public Health. 18 (4): 399–405. doi:10.1093/eurpub/ckn015. PMID 18367496.
- ^ Mu Z, Chen PL, Geng FH, Ren L, Gu WC, Ma JY, Peng L, Li QY (2017). "Synergistic effects of temperature and humidity on the symptoms of COPD patients". Int J Biometeorol. 61 (11): 1919–25. Bibcode:2017IJBm...61.1919M. doi:10.1007/s00484-017-1379-0. PMID 28567499. S2CID 25962322.
- ^ "Humidifiers: Ease skin, breathing symptoms". Mayo Clinic.
- ^ Noti JD, Blachere FM, McMillen CM, Lindsley WG, Kashon ML, Slaughter DR, Beezhold DH (2013). "High humidity leads to loss of infectious influenza virus from simulated coughs". PLOS ONE. 8 (2): e57485. Bibcode:2013PLoSO...857485N. doi:10.1371/journal.pone.0057485. PMC 3583861. PMID 23460865.
- ^ a b Gold Report 2021, pp. 92–96, Chapter 4: Management of stable COPD.
- ^ van Geffen WH, Slebos DJ, Herth FJ, Kemp SV, Weder W, Shah PL (April 2019). "Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis" (PDF). The Lancet. Respiratory Medicine. 7 (4): 313–324. doi:10.1016/S2213-2600(18)30431-4. PMID 30744937. S2CID 73428098.
- ^ a b Duffy S, Marchetti N, Criner GJ (September 2020). "Surgical Therapies for Chronic Obstructive Pulmonary Disease". Clinics in Chest Medicine. 41 (3): 559–566. doi:10.1016/j.ccm.2020.06.011. PMID 32800206. S2CID 221145423.
- ^ a b "1 Recommendations | Endobronchial valve insertion to reduce lung volume in emphysema | Guidance | NICE". www.nice.org.uk. 20 December 2017. Retrieved 7 July 2021.
- ^ Klooster K, Slebos DJ (May 2021). "Endobronchial Valves for the Treatment of Advanced Emphysema". Chest. 159 (5): 1833–1842. doi:10.1016/j.chest.2020.12.007. PMC 8129734. PMID 33345947.
- ^ Welling JB, Slebos DJ (August 2018). "Lung volume reduction with endobronchial coils for patients with emphysema". J Thorac Dis. 10 (Suppl 23): S2797–S2805. doi:10.21037/jtd.2017.12.95. PMC 6129816. PMID 30210833.
- ^ Valipour A (January 2017). "Bronchoscopic Thermal Vapour Ablation: Hot Stuff to Treat Emphysema Patients!". Archivos de Bronconeumologia. 53 (1): 1–2. doi:10.1016/j.arbr.2016.11.009. PMID 27916315. S2CID 78181696.
- ^ Mortensen J, Berg RM (January 2019). "Lung Scintigraphy in COPD". Seminars in Nuclear Medicine. 49 (1): 16–21. doi:10.1053/j.semnuclmed.2018.10.010. PMID 30545511. S2CID 56486118.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 2009-11-11. Retrieved Nov 11, 2009.
- ^ a b Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. (December 2012). "Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2197–2223. doi:10.1016/S0140-6736(12)61689-4. PMID 23245608. S2CID 205967479.
- ^ Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. (December 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2163–2196. doi:10.1016/S0140-6736(12)61729-2. PMC 6350784. PMID 23245607.
- ^ Gold Report 2021, pp. 26–33, Chapter 2:Diagnosis and assessment.
- ^ a b "COPD prevalence". NICE. Retrieved 18 July 2021.
- ^ Rycroft CE, Heyes A, Lanza L, Becker K (2012). "Epidemiology of chronic obstructive pulmonary disease: a literature review". International Journal of Chronic Obstructive Pulmonary Disease. 7: 457–94. doi:10.2147/COPD.S32330. PMC 3422122. PMID 22927753.
- ^ Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, Kazerooni E, Murray S, Criner GJ, Sin DD, Hogg J, Ries AL, Han M, Fishman AP, Make B, Hoffman EA, Mohsenifar Z, Wise R (April 2007). "Sex differences in severe pulmonary emphysema". American Journal of Respiratory and Critical Care Medicine. 176 (3): 243–252. doi:10.1164/rccm.200606-828OC. PMC 1994221. PMID 17431226.
- ^ "Basics About COPD". Chronic Obstructive Pulmonary Disease (COPD). Center for Disease Control. 9 June 2021. Retrieved 18 July 2021.
- ^ a b Torio CM, Andrews RM (2006). "National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011: Statistical Brief #160". Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. PMID 24199255. Archived from the original on 2017-03-14.
- ^ "The top 10 causes of death". Fact Sheets. World Health Organization.
- ^ a b c d e f Petty TL (2006). "The history of COPD". International Journal of Chronic Obstructive Pulmonary Disease. 1 (1): 3–14. doi:10.2147/copd.2006.1.1.3. PMC 2706597. PMID 18046898.
- ^ a b Wright JL, Churg A (2008). "Pathologic Features of Chronic Obstructive Pulmonary Disease: Diagnostic Criteria and Differential Diagnosis" (PDF). In Fishman A, Elias J, Fishman J, Grippi M, Senior R, Pack A (eds.). Fishman's Pulmonary Diseases and Disorders (4th ed.). McGraw-Hill. pp. 693–705. ISBN 978-0-07-164109-8. Archived from the original (PDF) on 2016-03-03. Retrieved 2008-11-11.
- ^ Woolcock A (1984). "The Search for Words to Describe the Bad Blowers". Chest. 85 (6): 73S–74S. doi:10.1378/chest.85.6_Supplement.73S.
- ^ Waldbott GL (1965). A struggle with Titans. Carlton Press. p. 6.
- ^ Fishman AP (May 2005). "One hundred years of chronic obstructive pulmonary disease". American Journal of Respiratory and Critical Care Medicine. 171 (9): 941–8. doi:10.1164/rccm.200412-1685OE. PMID 15849329.
- ^ "November is National COPD Awareness Month | NHLBI, NIH". www.nhlbi.nih.gov. Retrieved 21 July 2021.
- ^ a b Lomborg B (2013). Global problems, local solutions : costs and benefits. Cambridge University Press. p. 143. ISBN 978-1-107-03959-9.
- ^ Bloom D (2011). The Global Economic Burden of Noncommunicable Diseases (PDF). World Economic Forum. p. 24. Archived (PDF) from the original on 2015-02-04.
- ^ "NIH study shows hyaluronan is effective in treating chronic lung disease". National Institutes of Health (NIH). 1 February 2021. Retrieved 7 August 2021.
- ^ Poggi C, Mantovani S, Pecoraro Y, Carillo C, Bassi M, D'Andrilli A, Anile M, Rendina EA, Venuta F, Diso D (November 2018). "Bronchoscopic treatment of emphysema: an update". J Thorac Dis. 10 (11): 6274–6284. doi:10.21037/jtd.2018.10.43. PMC 6297441. PMID 30622803.
- ^ "A Prospective Safety and Feasibility Study of the RejuvenAir™ System Metered Cryospray Therapy for Chronic Bronchitis Patients". clinicaltrials.gov. 25 January 2021. Retrieved 16 August 2021.
- ^ Chen YT, Miao K, Zhou L, Xiong WN (June 2021). "Stem cell therapy for chronic obstructive pulmonary disease". Chin Med J (Engl). 134 (13): 1535–1545. doi:10.1097/CM9.0000000000001596. PMC 8280064. PMID 34250959.
- ^ "First UK patients get pioneering new treatment for serious lung disease | Royal Brompton & Harefield hospitals". www.rbht.nhs.uk. Retrieved 4 August 2021.
- ^ Gøtzsche PC, Johansen HK (September 2016). "Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease". The Cochrane Database of Systematic Reviews. 2016 (9): CD007851. doi:10.1002/14651858.CD007851.pub3. PMC 6457738. PMID 27644166.
- ^ Campos MA, Geraghty P, Holt G, Mendes E, Newby PR, Ma S, et al. (August 2019). "The Biological Effects of Double-Dose Alpha-1 Antitrypsin Augmentation Therapy. A Pilot Clinical Trial". American Journal of Respiratory and Critical Care Medicine. 200 (3): 318–326. doi:10.1164/rccm.201901-0010OC. PMC 6680306. PMID 30965011.
- ^ Nambiar S, Bong How S, Gummer J, Trengove R, Moodley Y (February 2020). "Metabolomics in chronic lung diseases". Respirology. 25 (2): 139–148. doi:10.1111/resp.13530. PMID 30907495.
- ^ McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A (July 2011). "Telehealthcare for chronic obstructive pulmonary disease" (PDF). The Cochrane Database of Systematic Reviews. 2012 (7): CD007718. doi:10.1002/14651858.CD007718.pub2. PMC 8939044. PMID 21735417.
- ^ Cheng J, Eroglu A (June 2021). "The Promising Effects of Astaxanthin on Lung Diseases". Adv Nutr. 12 (3): 850–864. doi:10.1093/advances/nmaa143. PMC 8166543. PMID 33179051.
- ^ Gruß I, McCreary GM, Ivlev I, Houlihan ME, Yawn BP, Pasquale C, et al. (December 2021). "Developing a patient-driven chronic obstructive pulmonary disease (COPD) research agenda in the U.S." Journal of Patient-Reported Outcomes. 5 (1): 126. doi:10.1186/s41687-021-00399-7. PMC 8643383. PMID 34865193.
- ^ Akers RM, Denbow DM (2008). Anatomy and Physiology of Domestic Animals. Wiley. p. 852. ISBN 978-1-118-70115-7.
- ^ Churg A, Wright JL (2007). "Animal models of cigarette smoke-induced chronic obstructive lung disease". Models of Exacerbations in Asthma and COPD. Contributions to Microbiology. Vol. 14. pp. 113–25. doi:10.1159/000107058. ISBN 978-3-8055-8332-9. PMID 17684336.
- ^ "Recurrent Airway Obstruction in Horses - Respiratory System". Veterinary Manual. Retrieved 7 July 2021.
- ^ Miller MS, Tilley LP, Smith FW (January 1989). "Cardiopulmonary disease in the geriatric dog and cat". The Veterinary Clinics of North America. Small Animal Practice. 19 (1): 87–102. doi:10.1016/S0195-5616(89)50007-X. PMID 2646821.
Works cited
[edit]- Global Strategy for Prevention, Diagnosis and Management of COPD: 2021 Report (PDF). Global Initiative for Chronic Obstructive Lung Disease. 25 November 2020. Retrieved 28 June 2021.
External links
[edit]- WHO fact sheet on COPD
- "COPD". MedlinePlus. U.S. National Library of Medicine.