Nuclear organization
This article may be too technical for most readers to understand. (May 2016) |

Nuclear organization refers to the spatial organization and dynamics of chromatin within a cell nucleus during interphase. There are many different levels and scales of nuclear organisation.
At the smallest scale, DNA is packaged into units called nucleosomes, which compacts DNA about 7-fold. In addition, nucleosomes protect DNA from damage and carry epigenetic information. Positions of nucleosomes determine accessibility of DNA to transcription factors.
At the intermediate scale, DNA looping can physically bring together DNA elements that would otherwise be separated by large distances. These interactions allow regulatory signals to cross over large genomic distances—for example, from enhancers to promoters.
At a larger scale, chromosomes are organised into two compartments labelled A ("active") and B ("inactive"), which are further subdivided into sub-compartments.[1] At the largest scale, entire chromosomes segregate into distinct regions called chromosome territories.
Chromosome organization is dynamic at all scales.[2][3] Individual nucleosomes undergo constant thermal motion and nucleosome breathing. At intermediate scales, an active process of loop extrusion creates dynamic loops and Topologically Associating Domains (TADs).
Importance
[edit]Each human cell contains around two metres of DNA, which must be tightly folded to fit inside the cell nucleus. However, in order for the cell to function, proteins must be able to access the sequence information contained within the DNA, in spite of its tightly-packed nature. Hence, the cell has a number of mechanisms in place to control how DNA is organized.[4]
Moreover, nuclear organization can play a role in establishing cell identity. Cells within an organism have near identical nucleic acid sequences, but often exhibit different phenotypes. One way in which this individuality occurs is through changes in genome architecture, which can alter the expression of different sets of genes.[5] These alterations can have a downstream effect on cellular functions such as cell cycle facilitation, DNA replication, nuclear transport, and alteration of nuclear structure. Controlled changes in nuclear organization are essential for proper cellular function.
History and methodology
[edit]The organization of chromosomes into distinct regions within the nucleus was first proposed in 1885 by Carl Rabl. Later in 1909, with the help of the microscopy technology at the time, Theodor Boveri coined the termed chromosome territories after observing that chromosomes occupy individually distinct nuclear regions.[6] Since then, mapping genome architecture has become a major topic of interest.
Over the last ten years, rapid methodological developments have greatly advanced understanding in this field.[4] Large-scale DNA organization can be assessed with DNA imaging using fluorescent tags, such as DNA Fluorescence in situ hybridization (FISH), and specialized microscopes.[7] Additionally, high-throughput sequencing technologies such as Chromosome Conformation Capture-based methods can measure how often DNA regions are in close proximity.[8] At the same time, progress in genome-editing techniques (such as CRISPR/Cas9, ZFNs, and TALENs) have made it easier to test the organizational function of specific DNA regions and proteins.[9] There is also growing interest in the rheological properties of the interchromosomal space, studied by the means of Fluorescence Correlation Spectroscopy and its variants.[10][11]
Architectural proteins
[edit]Architectural proteins regulate chromatin structure by establishing physical interactions between DNA elements.[12] These proteins tend to be highly conserved across a majority of eukaryotic species.[13][14]
In mammals, key architectural proteins include:
- Histones: DNA is wrapped around histones to form nucleosomes, which are basic units of chromatin structure. Each nucleosome consists of 8 histone protein subunits, around which roughly 147 DNA base pairs are wrapped in 1.67 left-handed turns. Nucleosomes provide about 7-fold initial linear compaction of DNA.[15] The concentration and specific composition of histones used can determine local chromatin structure. For example, euchromatin is a form of chromatin with low nucleosome concentration - here, the DNA is exposed, promoting interactions with gene expression, replication, and organizational machinery. In contrast, heterochromatin has high nucleosome concentration and is associated with repression of gene expression and replication, as the necessary proteins cannot interact with the DNA.
- Chromatin remodeling enzymes: These enzymes are responsible for promoting euchromatin or heterochromatin formation by a number of processes, particularly modifying histone tails or physically moving the nucleosomes. This in turn, helps regulate gene expression, replication, and how the chromatin interacts with architectural factors.[16] The list of chromatin remodeling enzymes is extensive and many have specific roles within the nucleus. For example, in 2016 Wiechens et al. identified two human enzymes, SNF2H and SNF2L, that are active in regulating CTCF binding and therefore affect genome organization and transcription of many genes.[17]
- CCCTC-binding factor (CTCF), or 11-zinc finger protein, is considered the most prominent player in linking genome organization with gene expression.[14] CTCF interacts with specific DNA sequences and a variety of other architectural proteins, chiefly cohesin[18] - these behaviours allow it to mediate DNA looping, thus acting as transcriptional repressor, activator, and insulator. Furthermore, CTCF is often found at self-interacting domain boundaries, and can anchor the chromatin to the nuclear lamina.[19] CTCF is also involved in V(D)J recombination.[20]
- Cohesin: The cohesin complex was initially discovered as a key player in mitosis, binding sister chromatids together to ensure proper segregation. However, cohesin has since been linked to many more functions within the cell.[21] It has been found to help facilitate DNA repair and recombination, meiotic chromosome pairing and orientation, chromosome condensation, DNA replication, gene expression, and genome architecture.[22] Cohesin is a heterodimer composed of the proteins SMC1 and SMC3 in combination with the SCC1 and SCC3 proteins. The entire complex is loaded onto DNA by the NIPBL-MAU2 complex in a ring-like fashion.[23]
Levels of nuclear organisation
[edit]Nucleosome fiber
[edit]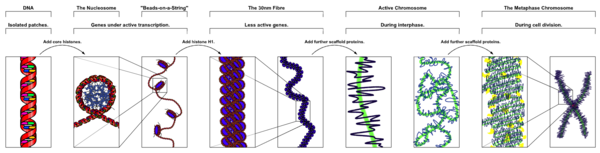
The organization of DNA within the nucleus begins with the 10 nm fiber, a "beads-on-a-string" structure[24] made of nucleosomes connected by 20-60bp linkers. A fiber of nucleosomes is interrupted by regions of accessible DNA, which are 100-1000bp long regions devoid of nucleosomes. Transcription factors bind within accessible DNA to displace nucleosomes and form cis-regulatory elements. Sites of accessible DNA are typically probed by ATAC-seq or DNase-Seq experimental methods.
A 30 nm fiber has long been proposed as the next layer of chromatin organization. While 30 nm fiber is often visible in vitro under high salt concentration,[25] its existence in vivo has been questioned in many recent studies.[26][27][28] Instead, these studies point towards a disordered fiber with a width of 20 to 50 nm.
Loop extrusion and DNA loops
[edit]
The process of loop extrusion by SMC complexes dynamically creates chromatin loops ranging in size from 50-100kb in yeast [29] to up to several Mb in mammals.[30] There is strong support for loop extrusion in yeast, mammals, and nematodes.[31]
In mammals, loop extrusion is responsible for the formation of topologically associating domains and loops between CTCF sites, as well as for bringing promoters and enhancers together. CTCF sites serve as boundaries of insulated neighborhoods or topologically associating domains.
The presence of loop extrusion in fruit flies is debated and the formation of DNA loops may be mediated by a different process of boundary element pairing.[32]
Chromosomal domains
[edit]Topologically associating domains
[edit]Self-interacting (or self-associating) domains are found in many organisms. In eukaryotes, they have been usually referred to as TADs irrespective of the mechanism of their formation. TADs have a higher ratio of chromosomal contacts within the domain than outside it.[33] They are formed through the help of architectural proteins. In many organisms, TADs correlate with regulation of gene expression, and enhancers and promoters within a TAD interact at higher frequency.[30]
Lamina-associating domains and nucleolar-associating domains
[edit]Lamina-associating domains (LADs) and nucleolar-associating domains (NADs) are regions of the chromosome that interact with the nuclear lamina and nucleolus, respectively.
Making up approximately 40% of the genome, LADs consist mostly of gene poor regions and span between 40kb to 30Mb in size.[19] There are two known types of LADs: constitutive LADs (cLADs) and facultative LADs (fLADs). cLADs are A-T rich heterochromatin regions that remain on lamina and are seen across many types of cells and species. There is evidence that these regions are important to the structural formation of interphase chromosome. On the other hand, fLADs have varying lamina interactions and contain genes that are either activated or repressed between individual cells indicating cell-type specificity.[34] The boundaries of LADs, like self-interacting domains, are enriched in transcriptional elements and architectural protein binding sites.[19]
NADs, which constitutes 4% of the genome, share nearly all of the same physical characteristics as LADs. In fact, DNA analysis of these two types of domains have shown that many sequences overlap, indicating that certain regions may switch between lamina-binding and nucleolus-binding.[35] NADs are associated with nucleolus function. The nucleolus is the largest sub-organelle within the nucleus and is the principal site for rRNA transcription. It also acts in signal recognition particle biosynthesis, protein sequestration, and viral replication.[36] The nucleolus forms around rDNA genes from different chromosomes. However, only a subset of rDNA genes is transcribed at a time and do so by looping into the interior of the nucleolus. The rest of the genes lay on the periphery of the sub-nuclear organelle in silenced heterochromatin state.[35]
A/B compartments
[edit]A/B compartments were first discovered in early Hi-C studies.[37][38] Researchers noticed that the whole genome could be split into two spatial compartments, labelled "A" and "B", where regions in compartment A tend to interact preferentially with A compartment-associated regions than B compartment-associated ones. Similarly, regions in compartment B tend to associate with other B compartment-associated regions.
A/B compartment-associated regions are on the multi-Mb scale and correlate with either open and expression-active chromatin ("A" compartments) or closed and expression-inactive chromatin ("B" compartments).[37] A compartments tend to be gene-rich, have high GC-content, contain histone markers for active transcription, and usually displace the interior of the nucleus. As well, they are typically made up of self-interacting domains and contain early replication origins. B compartments, on the other hand, tend to be gene-poor, compact, contain histone markers for gene silencing, and lie on the nuclear periphery. They consist mostly of LADs and contain late replication origins.[37] In addition, higher resolution Hi-C coupled with machine learning methods has revealed that A/B compartments can be refined into subcompartments.[39][40]
The fact that compartments self-interact is consistent with the idea that the nucleus localizes proteins and other factors such as long non-coding RNA (lncRNA) in regions suited for their individual roles.[citation needed] An example of this is the presence of multiple transcription factories throughout the nuclear interior.[41] These factories are associated with elevated levels of transcription due to the high concentration of transcription factors (such as transcription protein machinery, active genes, regulatory elements, and nascent RNA). Around 95% of active genes are transcribed within transcription factories. Each factory can transcribe multiple genes – these genes need not have similar product functions, nor do they need to lie on the same chromosome. Finally, the co-localization of genes within transcription factories is known to depend on cell type.[42]
Chromosome territories
[edit]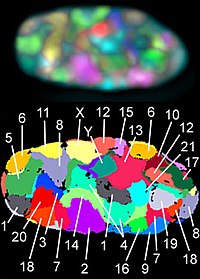
The last level of organization concerns the distinct positioning of individual chromosomes within the nucleus. The region occupied by a chromosome is called a chromosome territory (CT).[43] Among eukaryotes, CTs have several common properties. First, although chromosomal locations are not the same across cells within a population, there is some preference among individual chromosomes for particular regions. For example, large, gene-poor chromosomes are commonly located on the periphery near the nuclear lamina while smaller, gene-rich chromosomes group closer to the center of the nucleus.[44] Second, individual chromosome preference is variable among different cell types. For example, the X-chromosome has shown to localize to the periphery more often in liver cells than in kidney cells.[45] Another conserved property of chromosome territories is that homologous chromosomes tend to be far apart from one another during cell interphase. The final characteristic is that the position of individual chromosomes during each cell cycle stays relatively the same until the start of mitosis.[46] The mechanisms and reasons behind chromosome territory characteristics is still unknown and further experimentation is needed.
References
[edit]- ^ Oji, A.; Choubani, L.; Miura, H.; Hiratani, I. (October 2024). "Structure and dynamics of nuclear A/B compartments and subcompartments". Current Opinion in Cell Biology. 90: 102406. doi:10.1016/j.ceb.2024.102406. ISSN 1879-0410. PMID 39083950.
- ^ Johnstone, Christopher P.; Wang, Nathan B.; Sevier, Stuart A.; Galloway, Kate E. (18 November 2020). "Understanding and Engineering Chromatin as a Dynamical System across Length and Timescales". Cell Systems. 11 (5): 424–448. doi:10.1016/j.cels.2020.09.011. ISSN 2405-4720. PMID 33212016.
- ^ Jha, Rajiv Kumar; Levens, David; Kouzine, Fedor (December 2022). "Mechanical determinants of chromatin topology and gene expression". Nucleus. 13 (1): 94–115. doi:10.1080/19491034.2022.2038868. ISSN 1949-1042. PMC 8890386. PMID 35220881.
- ^ a b Fraser J, Williamson I, Bickmore WA, Dostie J (September 2015). "An Overview of Genome Organization and How We Got There: from FISH to Hi-C". Microbiology and Molecular Biology Reviews. 79 (3): 347–72. doi:10.1128/MMBR.00006-15. PMC 4517094. PMID 26223848.
- ^ Pombo A, Dillon N (April 2015). "Three-dimensional genome architecture: players and mechanisms". Nature Reviews. Molecular Cell Biology. 16 (4): 245–57. doi:10.1038/nrm3965. PMID 25757416. S2CID 6713103.
- ^ Cremer T, Cremer M, Hübner B, Strickfaden H, Smeets D, Popken J, et al. (October 2015). "The 4D nucleome: Evidence for a dynamic nuclear landscape based on co-aligned active and inactive nuclear compartments". FEBS Letters. 589 (20 Pt A): 2931–43. Bibcode:2015FEBSL.589.2931C. doi:10.1016/j.febslet.2015.05.037. PMID 26028501. S2CID 10254118.
- ^ Risca VI, Greenleaf WJ (July 2015). "Unraveling the 3D genome: genomics tools for multiscale exploration". Trends in Genetics. 31 (7): 357–72. doi:10.1016/j.tig.2015.03.010. PMC 4490074. PMID 25887733.
- ^ de Wit E, de Laat W (January 2012). "A decade of 3C technologies: insights into nuclear organization". Genes & Development. 26 (1): 11–24. doi:10.1101/gad.179804.111. PMC 3258961. PMID 22215806.
- ^ Gaj T, Gersbach CA, Barbas CF (July 2013). "ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering". Trends in Biotechnology. 31 (7): 397–405. doi:10.1016/j.tibtech.2013.04.004. PMC 3694601. PMID 23664777.
- ^ Bubak G, Kwapiszewska K, Kalwarczyk T, Bielec K, Andryszewski T, Iwan M, et al. (January 2021). "Quantifying Nanoscale Viscosity and Structures of Living Cells Nucleus from Mobility Measurements". The Journal of Physical Chemistry Letters. 12 (1): 294–301. doi:10.1021/acs.jpclett.0c03052. PMID 33346672. S2CID 229342582.
- ^ Baum M, Erdel F, Wachsmuth M, Rippe K (July 2014). "Retrieving the intracellular topology from multi-scale protein mobility mapping in living cells". Nature Communications. 5 (1): 4494. Bibcode:2014NatCo...5.4494B. doi:10.1038/ncomms5494. PMC 4124875. PMID 25058002.
- ^ Gómez-Díaz E, Corces VG (November 2014). "Architectural proteins: regulators of 3D genome organization in cell fate". Trends in Cell Biology. 24 (11): 703–11. doi:10.1016/j.tcb.2014.08.003. PMC 4254322. PMID 25218583.
- ^ Campos EI, Reinberg D (December 2009). "Histones: annotating chromatin". Annual Review of Genetics. 43 (1): 559–99. doi:10.1146/annurev.genet.032608.103928. PMID 19886812.
- ^ a b Ong CT, Corces VG (April 2014). "CTCF: an architectural protein bridging genome topology and function". Nature Reviews. Genetics. 15 (4): 234–46. doi:10.1038/nrg3663. PMC 4610363. PMID 24614316.
- ^ Cutter, A. R., Hayes, J. J. (7 October 2015). "A brief review of nucleosome structure". FEBS Letters. 589 (20 Pt A): 2914–2922. Bibcode:2015FEBSL.589.2914C. doi:10.1016/j.febslet.2015.05.016. ISSN 1873-3468. PMC 4598263. PMID 25980611.
- ^ Phillips, T. & Shaw, K. (2008) Chromatin Remodeling in Eukaryotes. Nature Education 1(1):209
- ^ Wiechens N, Singh V, Gkikopoulos T, Schofield P, Rocha S, Owen-Hughes T (March 2016). "The Chromatin Remodelling Enzymes SNF2H and SNF2L Position Nucleosomes adjacent to CTCF and Other Transcription Factors". PLOS Genetics. 12 (3): e1005940. doi:10.1371/journal.pgen.1005940. PMC 4809547. PMID 27019336.
- ^ Rubio ED, Reiss DJ, Welcsh PL, Disteche CM, Filippova GN, Baliga NS, et al. (June 2008). "CTCF physically links cohesin to chromatin". Proceedings of the National Academy of Sciences of the United States of America. 105 (24): 8309–14. Bibcode:2008PNAS..105.8309R. doi:10.1073/pnas.0801273105. PMC 2448833. PMID 18550811.
- ^ a b c Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, et al. (June 2008). "Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions". Nature. 453 (7197): 948–51. Bibcode:2008Natur.453..948G. doi:10.1038/nature06947. PMID 18463634. S2CID 4429401.
- ^ Chaumeil J, Skok JA (April 2012). "The role of CTCF in regulating V(D)J recombination". Current Opinion in Immunology. 24 (2): 153–9. doi:10.1016/j.coi.2012.01.003. PMC 3444155. PMID 22424610.
- ^ Peters JM, Tedeschi A, Schmitz J (November 2008). "The cohesin complex and its roles in chromosome biology". Genes & Development. 22 (22): 3089–114. doi:10.1101/gad.1724308. PMID 19056890.
- ^ Mehta GD, Kumar R, Srivastava S, Ghosh SK (August 2013). "Cohesin: functions beyond sister chromatid cohesion". FEBS Letters. 587 (15): 2299–312. Bibcode:2013FEBSL.587.2299M. doi:10.1016/j.febslet.2013.06.035. PMID 23831059. S2CID 39397443.
- ^ Nasmyth K, Haering CH (2009). "Cohesin: its roles and mechanisms". Annual Review of Genetics. 43: 525–58. doi:10.1146/annurev-genet-102108-134233. PMID 19886810.
- ^ Baldi, S., Korber, P., Becker, P. B. (February 2020). "Beads on a string-nucleosome array arrangements and folding of the chromatin fiber". Nature Structural & Molecular Biology. 27 (2): 109–118. doi:10.1038/s41594-019-0368-x. ISSN 1545-9985. PMID 32042149.
- ^ Song, F., Chen, P., Sun, D., Wang, M., Dong, L., Liang, D., Xu, R.-M., Zhu, P., Li, G. (25 April 2014). "Cryo-EM study of the chromatin fiber reveals a double helix twisted by tetranucleosomal units". Science. 344 (6182): 376–380. Bibcode:2014Sci...344..376S. doi:10.1126/science.1251413. ISSN 1095-9203. PMID 24763583.
- ^ Maeshima, K., Ide, S., Babokhov, M. (June 2019). "Dynamic chromatin organization without the 30-nm fiber". Current Opinion in Cell Biology. 58: 95–104. doi:10.1016/j.ceb.2019.02.003. ISSN 1879-0410. PMID 30908980.
- ^ Ou, H. D., Phan, S., Deerinck, T. J., Thor, A., Ellisman, M. H., O’Shea, C. C. (28 July 2017). "ChromEMT: Visualizing 3D chromatin structure and compaction in interphase and mitotic cells". Science. 357 (6349). doi:10.1126/science.aag0025. ISSN 0036-8075. PMC 5646685. PMID 28751582.
- ^ Hou, Z., Nightingale, F., Zhu, Y., MacGregor-Chatwin, C., Zhang, P. (10 October 2023). "Structure of native chromatin fibres revealed by Cryo-ET in situ". Nature Communications. 14 (1): 6324. Bibcode:2023NatCo..14.6324H. doi:10.1038/s41467-023-42072-1. ISSN 2041-1723. PMC 10564948. PMID 37816746.
- ^ Yuan, T., Yan, H., Li, K. C., Surovtsev, I., King, M. C., Mochrie, S. G. J. (14 November 2024). "Cohesin distribution alone predicts chromatin organization in yeast via conserved-current loop extrusion". Genome Biology. 25 (1): 293. doi:10.1186/s13059-024-03432-2. ISSN 1474-760X. PMC 11566905. PMID 39543681.
- ^ a b Karpinska, M. A., Oudelaar, A. M. (April 2023). "The role of loop extrusion in enhancer-mediated gene activation". Current Opinion in Genetics & Development. 79: 102022. ISSN 1879-0380.
- ^ Kim, J., Wang, H., Ercan, S. (2024), "Cohesin organizes 3D DNA contacts surrounding active enhancers in C. elegans", BioRxiv: The Preprint Server for Biology, doi:10.1101/2023.09.18.558239, PMC 10541618, PMID 37786717
- ^ Bing, Xinyang; Ke, Wenfan; Fujioka, Miki; Kurbidaeva, Amina; Levitt, Sarah; Levine, Mike; Schedl, Paul; Jaynes, James B (7 August 2024). "Chromosome structure in Drosophila is determined by boundary pairing not loop extrusion". eLife. 13. Mia T Levine, Claude Desplan (eds.): –94070. doi:10.7554/eLife.94070. ISSN 2050-084X. PMC 11305675. PMID 39110499.
- ^ Dixon, Jesse R.; Selvaraj, Siddarth; Yue, Feng; Kim, Audrey; Li, Yan; Shen, Yin; Hu, Ming; Liu, Jun S.; Ren, Bing (May 2012). "Topological domains in mammalian genomes identified by analysis of chromatin interactions". Nature. 485 (7398): 376–380. Bibcode:2012Natur.485..376D. doi:10.1038/nature11082. ISSN 1476-4687. PMC 3356448. PMID 22495300.
- ^ Meuleman W, Peric-Hupkes D, Kind J, Beaudry JB, Pagie L, Kellis M, et al. (February 2013). "Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence". Genome Research. 23 (2): 270–80. doi:10.1101/gr.141028.112. PMC 3561868. PMID 23124521.
- ^ a b van Koningsbruggen S, Gierlinski M, Schofield P, Martin D, Barton GJ, Ariyurek Y, et al. (November 2010). "High-resolution whole-genome sequencing reveals that specific chromatin domains from most human chromosomes associate with nucleoli". Molecular Biology of the Cell. 21 (21): 3735–48. doi:10.1091/mbc.E10-06-0508. PMC 2965689. PMID 20826608.
- ^ Matheson TD, Kaufman PD (June 2016). "Grabbing the genome by the NADs". Chromosoma. 125 (3): 361–71. doi:10.1007/s00412-015-0527-8. PMC 4714962. PMID 26174338.
- ^ a b c Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. (October 2009). "Comprehensive mapping of long-range interactions reveals folding principles of the human genome". Science. 326 (5950): 289–93. Bibcode:2009Sci...326..289L. doi:10.1126/science.1181369. PMC 2858594. PMID 19815776.
- ^ Fortin JP, Hansen KD (August 2015). "Reconstructing A/B compartments as revealed by Hi-C using long-range correlations in epigenetic data". Genome Biology. 16 (1): 180. doi:10.1186/s13059-015-0741-y. PMC 4574526. PMID 26316348.
- ^ Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, et al. (December 2014). "A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping". Cell. 159 (7): 1665–80. doi:10.1016/j.cell.2014.11.021. PMC 5635824. PMID 25497547.
- ^ Xiong K, Ma J (November 2019). "Revealing Hi-C subcompartments by imputing inter-chromosomal chromatin interactions". Nature Communications. 10 (1): 5069. Bibcode:2019NatCo..10.5069X. doi:10.1038/s41467-019-12954-4. PMC 6838123. PMID 31699985.
- ^ Cook PR (January 2010). "A model for all genomes: the role of transcription factories". Journal of Molecular Biology. 395 (1): 1–10. doi:10.1016/j.jmb.2009.10.031. PMID 19852969.
- ^ Buckley MS, Lis JT (April 2014). "Imaging RNA Polymerase II transcription sites in living cells". Current Opinion in Genetics & Development. 25: 126–30. doi:10.1016/j.gde.2014.01.002. PMC 5497218. PMID 24794700.
- ^ Cremer T, Cremer M (March 2010). "Chromosome territories". Cold Spring Harbor Perspectives in Biology. 2 (3): a003889. doi:10.1101/cshperspect.a003889. PMC 2829961. PMID 20300217.
- ^ Croft JA, Bridger JM, Boyle S, Perry P, Teague P, Bickmore WA (June 1999). "Differences in the localization and morphology of chromosomes in the human nucleus". The Journal of Cell Biology. 145 (6): 1119–31. doi:10.1083/jcb.145.6.1119. PMC 2133153. PMID 10366586.
- ^ Parada LA, McQueen PG, Misteli T (2004). "Tissue-specific spatial organization of genomes". Genome Biology. 5 (7): R44. doi:10.1186/gb-2004-5-7-r44. PMC 463291. PMID 15239829.
- ^ Walter J, Schermelleh L, Cremer M, Tashiro S, Cremer T (March 2003). "Chromosome order in HeLa cells changes during mitosis and early G1, but is stably maintained during subsequent interphase stages". The Journal of Cell Biology. 160 (5): 685–97. doi:10.1083/jcb.200211103. PMC 2173351. PMID 12604593.
External links
[edit] Media related to Nuclear organization at Wikimedia Commons
Media related to Nuclear organization at Wikimedia Commons
