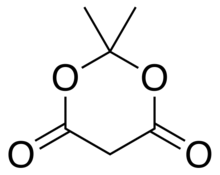
Meldrum's acid
| |
| Names | |
|---|---|
| IUPAC name
2,2-Dimethyl-1,3-dioxane-4,6-dione
| |
| Properties | |
| C6H8O4 | |
| Molar mass | 144.1253 g/mol |
| Density | ? g/cm3 |
| Melting point | 94-95 °C (decomposes)[1] |
| Acidity (pKa) | 4.97 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound. The compound was first made in 1908 by Andrew Norman Meldrum by a condensation reaction of malonic acid with acetone in acetic anhydride and sulfuric acid.[2] Meldrum misidentified the structure as a beta-lactone with a free carboxyl group. The correct structure[3] is shown on this page.
As an alternative to its original preparation, Meldrum's acid can be synthesized from malonic acid, isopropenyl acetate, and catalytic sulfuric acid. Meldrum's acid has a high acidity with a pKa of 4.97. Meldrum's acid high acidity was long considered "anomalous" given it is 8 orders of magnitude more acidic than the highly related compound, dimethyl malonate (whose aqueous pKa is around 13). In 2004, Ohwada and coworkers resolved the Meldrum acid anomaly by performing several calculations. [4] Ohwada noticed that the energy-minimizing conformation structure of the compound places the alpha proton's orbital in the proper geometry to align with the , so that the ground state poses unusually strong destabilization of the C-H bond.
Because of its great acidity, Meldrum's acid, like malonic acid, can serve as a reactant in Knoevenagel condensations.
Meldrum's acid is the basis for a general synthesis of beta-ketoesters. Reaction of Meldrum's acid with a carboxylic acid chloride (RCOCl) in dichloromethane-pyridine yields the corresponding acyl-Meldrum's Acid. When the latter is refluxed with an aliphatic alcohol (R'OH), a ketoester RCOCH2COOR' is the result, often in better than 80% overall yield, even with reactants as hindered as tertiary-butanol.[5] Ketoesters of this type are useful in the Knorr pyrrole synthesis.
References
- ^ "Meldrum's Acid". The Merck Index. Vol. 14th edition. Merck Research Laboratories. 2006. p. 1005. ISBN 978-0-911910-00-1.
- ^ Meldrum, Andrew Norman (1908). "A β-lactonic acid from acetone and malonic acid". Journal of the Chemical Society, Transactions. 93: 598–601. doi:10.1039/CT9089300598.
- ^ Davidson, David (1948). "The Structure of Meldrum's Supposed β-Lactonic Acid". Journal of the American Chemical Society. 70 (10): 3426–3428. doi:10.1021/ja01190a060.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Nakamura, Satoshi; Hirao, Hajime; Ohwada, Tomohiko (2004). "Rationale for the Acidity of Meldrum's Acid. Consistent Relation of C−H Acidities to the Properties of Localized Reactive Orbital". Journal of Organic Chemistry. 69: 4309–4316.
- ^ Oikawa, Yuji; Sugano, Kiyoshi; Yonemitsu, Osamu (1978). "Meldrum's acid in organic synthesis. 2. A general and versatile synthesis of β-keto esters". Journal of Organic Chemistry. 43: 2087–2088.
Further reading
- Kidd, Hamish (2008). "Meldrum's Acid". Chemistry World: 35–36.
{{cite journal}}: Unknown parameter|month=ignored (help)
- Lipson VV & Gorobets NY (2009). "One hundred years of Meldrum's acid: advances in the synthesis of pyridine and pyrimidine derivatives". Molecular Diversity. 13: 339–419. doi:10.1007/s11030-009-9136-x.
- McNab, Hamish (1978). "Meldrum's Acid". Chemical Society Reviews: 345–358. doi:10.1039/CS9780700345.
External links
- Synlett Spotlight Website
- Meldrum's acid in Organic Syntheses Website


