Manganese
 Pure manganese cube and oxidized manganese chips | |||||||||||||||||||||||||||||||||||
| Manganese | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈmæŋɡəniːz/ | ||||||||||||||||||||||||||||||||||
| Appearance | silvery metallic | ||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Mn) | |||||||||||||||||||||||||||||||||||
| Manganese in the periodic table | |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 25 | ||||||||||||||||||||||||||||||||||
| Group | group 7 | ||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d5 4s2 | ||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 13, 2 | ||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||
| Melting point | 1519 K (1246 °C, 2275 °F) | ||||||||||||||||||||||||||||||||||
| Boiling point | 2334 K (2061 °C, 3742 °F) | ||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 7.476 g/cm3 [3] | ||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 5.95 g/cm3 | ||||||||||||||||||||||||||||||||||
| Heat of fusion | 12.91 kJ/mol | ||||||||||||||||||||||||||||||||||
| Heat of vaporization | 221 kJ/mol | ||||||||||||||||||||||||||||||||||
| Molar heat capacity | 26.32 J/(mol·K) | ||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||
| Oxidation states | common: +2, +4, +7 −3,[4] −2,[5][4] −1,[4] 0,[4] +1,[4] +3,[4] +5,[4] +6[4] | ||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.55 | ||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 127 pm | ||||||||||||||||||||||||||||||||||
| Covalent radius | Low spin: 139±5 pm High spin: 161±8 pm | ||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||
| Crystal structure | α-Mn: body-centered cubic (bcc) (cI58) | ||||||||||||||||||||||||||||||||||
| Lattice constant | a = 891.16 pm (at 20 °C)[3] | ||||||||||||||||||||||||||||||||||
| Thermal expansion | 23.61×10−6/K (at 20 °C)[3] | ||||||||||||||||||||||||||||||||||
| Thermal conductivity | 7.81 W/(m⋅K) | ||||||||||||||||||||||||||||||||||
| Electrical resistivity | 1.44 µΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | (α) +529.0×10−6 cm3/mol (293 K)[6] | ||||||||||||||||||||||||||||||||||
| Young's modulus | 198 GPa | ||||||||||||||||||||||||||||||||||
| Bulk modulus | 120 GPa | ||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 5150 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.0 | ||||||||||||||||||||||||||||||||||
| Brinell hardness | 196 MPa | ||||||||||||||||||||||||||||||||||
| CAS Number | 7439-96-5 | ||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||
| Discovery | Carl Wilhelm Scheele (1774) | ||||||||||||||||||||||||||||||||||
| First isolation | Johann Gottlieb Gahn (1774) | ||||||||||||||||||||||||||||||||||
| Isotopes of manganese | |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
Manganese is a chemical element; it has symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent; as a rubber additive; and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide.
Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes.[8] It is found mostly in the bones, but also the liver, kidneys, and brain.[9] In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes.
It is familiar in the laboratory in the form of the deep violet salt potassium permanganate. It occurs at the active sites in some enzymes.[10] Of particular interest is the use of a Mn-O cluster, the oxygen-evolving complex, in the production of oxygen by plants.
Characteristics
[edit]Physical properties
[edit]Manganese is a silvery-gray metal that resembles iron. It is hard and very brittle, difficult to fuse, but easy to oxidize.[11] Manganese and its common ions are paramagnetic.[12] Manganese tarnishes slowly in air and oxidizes ("rusts") like iron in water containing dissolved oxygen.[13]
Isotopes
[edit]Naturally occurring manganese is composed of one stable isotope, 55Mn. Several radioisotopes have been isolated and described, ranging in atomic weight from 46 u (46Mn) to 72 u (72Mn). The most stable are 53Mn with a half-life of 3.7 million years, 54Mn with a half-life of 312.2 days, and 52Mn with a half-life of 5.591 days. All of the remaining radioactive isotopes have half-lives of less than three hours, and the majority of less than one minute. The primary decay mode in isotopes lighter than the most abundant stable isotope, 55Mn, is electron capture and the primary mode in heavier isotopes is beta decay.[14] Manganese also has three meta states.[14]
Manganese is part of the iron group of elements, which are thought to be synthesized in large stars shortly before the supernova explosion.[15] 53Mn decays to 53Cr with a half-life of 3.7 million years. Because of its relatively short half-life, 53Mn is relatively rare, produced by cosmic rays impact on iron.[16] Manganese isotopic contents are typically combined with chromium isotopic contents and have found application in isotope geology and radiometric dating. Mn–Cr isotopic ratios reinforce the evidence from 26Al and 107Pd for the early history of the Solar System. Variations in 53Cr/52Cr and Mn/Cr ratios from several meteorites suggest an initial 53Mn/55Mn ratio, which indicate that Mn–Cr isotopic composition must result from in situ decay of 53Mn in differentiated planetary bodies. Hence, 53Mn provides additional evidence for nucleosynthetic processes immediately before coalescence of the Solar System.[17]
Allotropes
[edit] |
 |
Four allotropes (structural forms) of solid manganese are known, labeled α, β, γ and δ, and occurring at successively higher temperatures. All are metallic, stable at standard pressure, and have a cubic crystal lattice, but they vary widely in their atomic structures.[18][19][20]
Alpha manganese (α-Mn) is the equilibrium phase at room temperature. It has a body-centered cubic lattice and is unusual among elemental metals in having a very complex unit cell, with 58 atoms per cell (29 atoms per primitive unit cell) in four different types of site.[21][18] It is paramagnetic at room temperature and antiferromagnetic at temperatures below 95 K (−178 °C).[22]

Beta manganese (β-Mn) forms when heated above the transition temperature of 973 K (700 °C; 1,290 °F). It has a primitive cubic structure with 20 atoms per unit cell at two types of sites, which is as complex as that of any other elemental metal.[23] It is easily obtained as a metastable phase at room temperature by rapid quenching. It does not show magnetic ordering, remaining paramagnetic down to the lowest temperature measured (1.1 K).[23][24][25]
Gamma manganese (γ-Mn) forms when heated above 1,370 K (1,100 °C; 2,010 °F). It has a simple face-centered cubic structure (four atoms per unit cell). When quenched to room temperature it converts to β-Mn, but it can be stabilized at room temperature by alloying it with at least 5 percent of other elements (such as C, Fe, Ni, Cu, Pd or Au), and these solute-stabilized alloys distort into a face-centered tetragonal structure.[26][25]
Delta manganese (δ-Mn) forms when heated above 1,406 K (1,130 °C; 2,070 °F) and is stable up to the manganese melting point of 1,519 K (1,250 °C; 2,270 °F). It has a body-centered cubic structure (two atoms per cubic unit cell).[19][25]
Chemical compounds
[edit]
Common oxidation states of manganese are +2, +3, +4, +6, and +7, although all oxidation states from −3 to +7 have been observed. Manganese in oxidation state +7 is represented by salts of the intensely purple permanganate anion MnO−4. Potassium permanganate is a commonly used laboratory reagent because of its oxidizing properties; it is used as a topical medicine (for example, in the treatment of fish diseases). Solutions of potassium permanganate were among the first stains and fixatives to be used in the preparation of biological cells and tissues for electron microscopy.[28]
Aside from various permanganate salts, Mn(VII) is represented by the unstable, volatile derivative Mn2O7. Oxyhalides (MnO3F and MnO3Cl) are powerful oxidizing agents.[11] The most prominent example of Mn in the +6 oxidation state is the green anion manganate, [MnO4]2−. Manganate salts are intermediates in the extraction of manganese from its ores. Compounds with oxidation states +5 are somewhat elusive, and often found associated to an oxide (O2−) or nitride (N3−) ligand.[29] One example is the blue anion hypomanganate [MnO4]3−.[30]
Mn(IV) is somewhat enigmatic because it is common in nature but far rarer in synthetic chemistry. The most common Mn ore, pyrolusite, is MnO2. It is the dark brown pigment of many cave drawings[31] but is also a common ingredient in dry cell batteries.[32] Complexes of Mn(IV) are well known, but they require elaborate ligands. Mn(IV)-OH complexes are an intermediate in some enzymes, including the oxygen evolving center (OEC) in plants.[33]
Simple derivatives Mn3+ are rarely encountered but can be stabilized by suitably basic ligands. Manganese(III) acetate is an oxidant useful in organic synthesis. Solid compounds of manganese(III) are characterized by its strong purple-red color and a preference for distorted octahedral coordination resulting from the Jahn-Teller effect.[34]

A particularly common oxidation state for manganese in aqueous solution is +2, which has a pale pink color. Many manganese(II) compounds are known, such as the aquo complexes derived from manganese(II) sulfate (MnSO4) and manganese(II) chloride (MnCl2). This oxidation state is also seen in the mineral rhodochrosite (manganese(II) carbonate). Manganese(II) commonly exists with a high spin, S = 5/2 ground state because of the high pairing energy for manganese(II). There are no spin-allowed d–d transitions in manganese(II), which explain its faint color.[35]
| Oxidation states of manganese[36] | |
|---|---|
| −3 | Mn(CO)(NO) 3 |
| −2 | [Mn(1,5-COD)2]2− |
| −1 | HMn(CO) 5 |
| 0 | Mn 2(CO) 10 |
| +1 | MnC 5H 4CH 3(CO) 3 |
| +2 | MnCl 2, MnCO 3, MnO |
| +3 | MnF 3, Mn(OAc) 3, Mn 2O 3 |
| +4 | MnO 2 |
| +5 | K 3MnO 4 |
| +6 | K 2MnO 4 |
| +7 | KMnO 4, Mn 2O 7 |
| Common oxidation states are in bold. | |
Organomanganese compounds
[edit]Manganese forms a large variety of organometallic derivatives, i.e., compounds with Mn-C bonds. The organometallic derivatives include numerous examples of Mn in its lower oxidation states, i.e. Mn(−III) up through Mn(I). This area of organometallic chemistry is attractive because Mn is inexpensive and of relatively low toxicity.[37]
Of greatest commercial interest is "MMT", methylcyclopentadienyl manganese tricarbonyl, which is used as an anti-knock compound added to gasoline (petrol) in some countries. It features Mn(I). Consistent with other aspects of Mn(II) chemistry, manganocene (Mn(C5H5)2) is high-spin. In contrast, its neighboring metal iron forms an air-stable, low-spin derivative in the form of ferrocene (Fe(C5H5)2). When conducted under an atmosphere of carbon monoxide, reduction of Mn(II) salts gives dimanganese decacarbonyl Mn2(CO)10, an orange and volatile solid. The air-stability of this Mn(0) compound (and its many derivatives) reflects the powerful electron-acceptor properties of carbon monoxide. Many alkene complexes and alkyne complexes are derived from Mn2(CO)10.[citation needed]
In Mn(CH3)2(dmpe)2, Mn(II) is low spin, which contrasts with the high spin character of its precursor, MnBr2(dmpe)2 (dmpe = (CH3)2PCH2CH2P(CH3)2).[38] Polyalkyl and polyaryl derivatives of manganese often exist in higher oxidation states, reflecting the electron-releasing properties of alkyl and aryl ligands. One example is [Mn(CH3)6]2−.[citation needed]
History
[edit]The origin of the name manganese is complex. In ancient times, two black minerals were identified from the regions of the Magnetes (either Magnesia, located within modern Greece, or Magnesia ad Sipylum, located within modern Turkey).[39] They were both called magnes from their place of origin, but were considered to differ in sex. The male magnes attracted iron, and was the iron ore now known as lodestone or magnetite, and which probably gave us the term magnet. The female magnes ore did not attract iron, but was used to decolorize glass. This female magnes was later called magnesia, known now in modern times as pyrolusite or manganese dioxide.[40] Neither this mineral nor elemental manganese is magnetic. In the 16th century, manganese dioxide was called manganesum (note the two Ns instead of one) by glassmakers, possibly as a corruption and concatenation of two words, since alchemists and glassmakers eventually had to differentiate a magnesia nigra (the black ore) from magnesia alba (a white ore, also from Magnesia, also useful in glassmaking). Michele Mercati called magnesia nigra manganesa, and finally the metal isolated from it became known as manganese (German: Mangan). The name magnesia eventually was then used to refer only to the white magnesia alba (magnesium oxide), which provided the name magnesium for the free element when it was isolated much later.[41]

Manganese dioxide, which is abundant in nature, has long been used as a pigment. The cave paintings in Gargas that are 30,000 to 24,000 years old are made from the mineral form of MnO2 pigments.[43]
Manganese compounds were used by Egyptian and Roman glassmakers, either to add to, or remove, color from glass.[44] Use as "glassmakers soap" continued through the Middle Ages until modern times and is evident in 14th-century glass from Venice.[45]

Because it was used in glassmaking, manganese dioxide was available for experiments by alchemists, the first chemists. Ignatius Gottfried Kaim (1770) and Johann Glauber (17th century) discovered that manganese dioxide could be converted to permanganate, a useful laboratory reagent.[46] Kaim also may have reduced manganese dioxide to isolate the metal, but that is uncertain.[47] By the mid-18th century, the Swedish chemist Carl Wilhelm Scheele used manganese dioxide to produce chlorine. First, hydrochloric acid, or a mixture of dilute sulfuric acid and sodium chloride was made to react with manganese dioxide, and later hydrochloric acid from the Leblanc process was used and the manganese dioxide was recycled by the Weldon process. The production of chlorine and hypochlorite bleaching agents was a large consumer of manganese ores.[citation needed]
Scheele and others were aware that pyrolusite (mineral form of manganese dioxide) contained a new element. Johan Gottlieb Gahn isolated an impure sample of manganese metal in 1774, which he did by reducing the dioxide with carbon.[48]
The manganese content of some iron ores used in Greece led to speculations that steel produced from that ore contains additional manganese, making the Spartan steel exceptionally hard.[49] Around the beginning of the 19th century, manganese was used in steelmaking and several patents were granted. In 1816, it was documented that iron alloyed with manganese was harder but not more brittle. In 1837, British academic James Couper noted an association between miners' heavy exposure to manganese and a form of Parkinson's disease.[50] In 1912, United States patents were granted for protecting firearms against rust and corrosion with manganese phosphate electrochemical conversion coatings, and the process has seen widespread use ever since.[51]
The invention of the Leclanché cell in 1866 and the subsequent improvement of batteries containing manganese dioxide as cathodic depolarizer increased the demand for manganese dioxide. Until the development of batteries with nickel–cadmium and lithium, most batteries contained manganese. The zinc–carbon battery and the alkaline battery normally use industrially produced manganese dioxide because naturally occurring manganese dioxide contains impurities. In the 20th century, manganese dioxide was widely used as the cathodic for commercial disposable dry batteries of both the standard (zinc–carbon) and alkaline types.[52]
Manganese is essential to iron and steel production by virtue of its sulfur-fixing, deoxidizing, and alloying properties.[53] This application was first recognized by the British metallurgist Robert Forester Mushet (1811–1891) who, in 1856, introduced the element, in the form of Spiegeleisen.
Occurrence
[edit]Manganese comprises about 1000 ppm (0.1%) of the Earth's crust and is the 12th most abundant element.[9] Soil contains 7–9000 ppm of manganese with an average of 440 ppm.[9] The atmosphere contains 0.01 μg/m3.[9] Manganese occurs principally as pyrolusite (MnO2), braunite (Mn2+Mn3+6)SiO12),[54] psilomelane (Ba,H2O)2Mn5O10, and to a lesser extent as rhodochrosite (MnCO3).
 |
 |
 |
 |
 |
| Manganese ore | Psilomelane (manganese ore) | Spiegeleisen is an iron alloy with a manganese content of approximately 15%. | Manganese oxide dendrites on limestone from Solnhofen, Germany – a kind of pseudofossil. Scale is in mm | Mineral rhodochrosite (manganese(II) carbonate) |

The most important manganese ore is pyrolusite (MnO2). Other economically important manganese ores usually show a close spatial relation to the iron ores, such as sphalerite.[11][55] Land-based resources are large but irregularly distributed. About 80% of the known world manganese resources are in South Africa; other important manganese deposits are in Ukraine, Australia, India, China, Gabon and Brazil.[53] According to 1978 estimate, the ocean floor has 500 billion tons of manganese nodules.[56] Attempts to find economically viable methods of harvesting manganese nodules were abandoned in the 1970s.[57]
In South Africa, most identified deposits are located near Hotazel in the Northern Cape Province, (Kalahari manganese fields), with a 2011 estimate of 15 billion tons. In 2011 South Africa produced 3.4 million tons, topping all other nations.[58]
Manganese is mainly mined in South Africa, Australia, China, Gabon, Brazil, India, Kazakhstan, Ghana, Ukraine and Malaysia.[59]
Production
[edit]For the production of ferromanganese, the manganese ore is mixed with iron ore and carbon, and then reduced either in a blast furnace or in an electric arc furnace.[60] The resulting ferromanganese has a manganese content of 30–80%.[11] Pure manganese used for the production of iron-free alloys is produced by leaching manganese ore with sulfuric acid and a subsequent electrowinning process.[61]
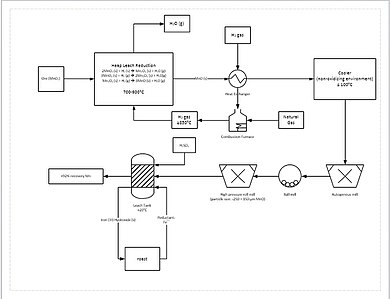
A more progressive extraction process involves directly reducing (a low grade) manganese ore by heap leaching. This is done by percolating natural gas through the bottom of the heap; the natural gas provides the heat (needs to be at least 850 °C) and the reducing agent (carbon monoxide). This reduces all of the manganese ore to manganese oxide (MnO), which is a leachable form. The ore then travels through a grinding circuit to reduce the particle size of the ore to between 150 and 250 μm, increasing the surface area to aid leaching. The ore is then added to a leach tank of sulfuric acid and ferrous iron (Fe2+) in a 1.6:1 ratio. The iron reacts with the manganese dioxide (MnO2) to form iron hydroxide (FeO(OH)) and elemental manganese (Mn).[citation needed]
This process yields approximately 92% recovery of the manganese. For further purification, the manganese can then be sent to an electrowinning facility.[62]
Oceanic environment
[edit]In 1972, the CIA's Project Azorian, through billionaire Howard Hughes, commissioned the ship Hughes Glomar Explorer with the cover story of harvesting manganese nodules from the sea floor.[63] That triggered a rush of activity to collect manganese nodules, which was not actually practical until the 2020s. The real mission of Hughes Glomar Explorer was to raise a sunken Soviet submarine, the K-129, with the goal of retrieving Soviet code books.[64]
An abundant resource of manganese in the form of manganese nodules found on the ocean floor.[65] These nodules, which are composed of 29% manganese,[66] are located along the ocean floor. The environmental impacts of nodule collection are of interest.[67][68]
Dissolved manganese (dMn) is found throughout the world's oceans, 90% of which originates from hydrothermal vents.[69] Particulate Mn develops in buoyant plumes over an active vent source, while the dMn behaves conservatively.[70] Mn concentrations vary between the water columns of the ocean. At the surface, dMn is elevated due to input from external sources such as rivers, dust, and shelf sediments. Coastal sediments normally have lower Mn concentrations, but can increase due to anthropogenic discharges from industries such as mining and steel manufacturing, which enter the ocean from river inputs. Surface dMn concentrations can also be elevated biologically through photosynthesis and physically from coastal upwelling and wind-driven surface currents. Internal cycling such as photo-reduction from UV radiation can also elevate levels by speeding up the dissolution of Mn-oxides and oxidative scavenging, preventing Mn from sinking to deeper waters.[71] Elevated levels at mid-depths can occur near mid-ocean ridges and hydrothermal vents. The hydrothermal vents release dMn enriched fluid into the water. The dMn can then travel up to 4,000 km due to the microbial capsules present, preventing exchange with particles, lowing the sinking rates. Dissolved Mn concentrations are even higher when oxygen levels are low. Overall, dMn concentrations are normally higher in coastal regions and decrease when moving offshore.[71]
Soils
[edit]Manganese occurs in soils in three oxidation states: the divalent cation, Mn2+ and as brownish-black oxides and hydroxides containing Mn (III,IV), such as MnOOH and MnO2. Soil pH and oxidation-reduction conditions affect which of these three forms of Mn is dominant in a given soil. At pH values less than 6 or under anaerobic conditions, Mn(II) dominates, while under more alkaline and aerobic conditions, Mn(III,IV) oxides and hydroxides predominate. These effects of soil acidity and aeration state on the form of Mn can be modified or controlled by microbial activity. Microbial respiration can cause both the oxidation of Mn2+ to the oxides, and it can cause reduction of the oxides to the divalent cation.[72]
The Mn(III,IV) oxides exist as brownish-black stains and small nodules on sand, silt, and clay particles. These surface coatings on other soil particles have high surface area and carry negative charge. The charged sites can adsorb and retain various cations, especially heavy metals (e.g., Cr3+, Cu2+, Zn2+, and Pb2+). In addition, the oxides can adsorb organic acids and other compounds. The adsorption of the metals and organic compounds can then cause them to be oxidized while the Mn(III,IV) oxides are reduced to Mn2+ (e.g., Cr3+ to Cr(VI) and colorless hydroquinone to tea-colored quinone polymers).[73]
Applications
[edit]Steel
[edit]
Manganese is essential to iron and steel production by virtue of its sulfur-fixing, deoxidizing, and alloying properties. Manganese has no satisfactory substitute in these applications in metallurgy.[53] Steelmaking,[74] including its ironmaking component, has accounted for most manganese demand, presently in the range of 85% to 90% of the total demand.[61] Manganese is a key component of low-cost stainless steel.[75][76] Often ferromanganese (usually about 80% manganese) is the intermediate in modern processes.
Small amounts of manganese improve the workability of steel at high temperatures by forming a high-melting sulfide and preventing the formation of a liquid iron sulfide at the grain boundaries. If the manganese content reaches 4%, the embrittlement of the steel becomes a dominant feature. The embrittlement decreases at higher manganese concentrations and reaches an acceptable level at 8%. Steel containing 8 to 15% of manganese has a high tensile strength of up to 863 MPa.[77][78] Steel with 12% manganese was discovered in 1882 by Robert Hadfield and is still known as Hadfield steel (mangalloy). It was used for British military steel helmets and later by the U.S. military.[79]
Aluminium alloys
[edit]Manganese is used in production of alloys with aluminium. Aluminium with roughly 1.5% manganese has increased resistance to corrosion through grains that absorb impurities which would lead to galvanic corrosion.[80] The corrosion-resistant aluminium alloys 3004 and 3104 (0.8 to 1.5% manganese) are used for most beverage cans.[81] Before 2000, more than 1.6 million tonnes of those alloys were used; at 1% manganese, this consumed 16,000 tonnes of manganese.[failed verification][81]
Batteries
[edit]Manganese(IV) oxide was used in the original type of dry cell battery as an electron acceptor from zinc, and is the blackish material in carbon–zinc type flashlight cells. The manganese dioxide is reduced to the manganese oxide-hydroxide MnO(OH) during discharging, preventing the formation of hydrogen at the anode of the battery.[82]
- MnO2 + H2O + e− → MnO(OH) + OH−
The same material also functions in newer alkaline batteries (usually battery cells), which use the same basic reaction, but a different electrolyte mixture. In 2002, more than 230,000 tons of manganese dioxide was used for this purpose.[52][82]

Resistors
[edit]Copper alloys of manganese, such as Manganin, are commonly found in metal element shunt resistors used for measuring relatively large amounts of current. These alloys have very low temperature coefficient of resistance and are resistant to sulfur. This makes the alloys particularly useful in harsh automotive and industrial environments.[83]
Fertilizers and feed additive
[edit]Manganese oxide and sulfate are components of fertilizers. In the year 2000, an estimated 20,000 tons of these compounds were used in fertilizers in the US alone. A comparable amount of Mn compounds was also used in animal feeds.[84]
Niche
[edit]Methylcyclopentadienyl manganese tricarbonyl is an additive in some unleaded gasoline to boost octane rating and reduce engine knocking.[85]
Manganese(IV) oxide (manganese dioxide, MnO2) is used as a reagent in organic chemistry for the oxidation of benzylic alcohols (where the hydroxyl group is adjacent to an aromatic ring). Manganese dioxide has been used since antiquity to oxidize and neutralize the greenish tinge in glass from trace amounts of iron contamination.[45] MnO2 is also used in the manufacture of oxygen and chlorine and in drying black paints. In some preparations, it is a brown pigment for paint and is a constituent of natural umber.[86]
Tetravalent manganese is used as an activator in red-emitting phosphors. While many compounds are known which show luminescence,[87] the majority are not used in commercial application due to low efficiency or deep red emission.[88][89] However, several Mn4+ activated fluorides were reported as potential red-emitting phosphors for warm-white LEDs.[90][91] But to this day, only K2SiF6:Mn4+ is commercially available for use in warm-white LEDs.[92]
The metal is occasionally used in coins; until 2000, the only United States coin to use manganese was the "wartime" nickel from 1942 to 1945.[93] An alloy of 75% copper and 25% nickel was traditionally used for the production of nickel coins. However, because of shortage of nickel metal during the war, it was substituted by more available silver and manganese, thus resulting in an alloy of 56% copper, 35% silver and 9% manganese. Since 2000, dollar coins, for example the Sacagawea dollar and the Presidential $1 coins, are made from a brass containing 7% of manganese with a pure copper core.[94] In both cases of nickel and dollar, the use of manganese in the coin was to duplicate the electromagnetic properties of a previous identically sized and valued coin in the mechanisms of vending machines. In the case of the later U.S. dollar coins, the manganese alloy was intended to duplicate the properties of the copper/nickel alloy used in the previous Susan B. Anthony dollar.
Manganese compounds have been used as pigments and for the coloring of ceramics and glass. The brown color of ceramic is sometimes the result of manganese compounds.[95] In the glass industry, manganese compounds are used for two effects. Manganese(III) reacts with iron(II) to reduce strong green color in glass by forming less-colored iron(III) and slightly pink manganese(II), compensating for the residual color of the iron(III).[45] Larger quantities of manganese are used to produce pink colored glass. In 2009, Mas Subramanian and associates at Oregon State University discovered that manganese can be combined with yttrium and indium to form an intensely blue, non-toxic, inert, fade-resistant pigment, YInMn Blue,[96] the first new blue pigment discovered in 200 years.[97]
Biochemistry
[edit]
Many classes of enzymes contain manganese cofactors including oxidoreductases, transferases, hydrolases, lyases, isomerases and ligases. Other enzymes containing manganese are arginase and a Mn-containing superoxide dismutase (Mn-SOD). Some reverse transcriptases of many retroviruses (although not lentiviruses such as HIV) contain manganese. Manganese-containing polypeptides are the diphtheria toxin, lectins, and integrins.[98]
The oxygen-evolving complex (OEC), containing four atoms of manganese, is a part of photosystem II contained in the thylakoid membranes of chloroplasts. The OEC is responsible for the terminal photooxidation of water during the light reactions of photosynthesis, i.e., it is the catalyst that makes the O2 produced by plants.[99][100]
Human health and nutrition
[edit]Manganese is an essential human dietary element and is present as a coenzyme in several biological processes, which include macronutrient metabolism, bone formation, and free radical defense systems. Manganese is a critical component in dozens of proteins and enzymes.[8] The human body contains about 12 mg of manganese, mostly in the bones. The soft tissue remainder is concentrated in the liver and kidneys.[9] In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes.[101]
| Males | Females | ||
|---|---|---|---|
| Age | AI (mg/day) | Age | AI (mg/day) |
| 1–3 | 1.2 | 1–3 | 1.2 |
| 4–8 | 1.5 | 4–8 | 1.5 |
| 9–13 | 1.9 | 9–13 | 1.6 |
| 14–18 | 2.2 | 14–18 | 1.6 |
| 19+ | 2.3 | 19+ | 1.8 |
| pregnant: 2 | |||
| lactating: 2.6 | |||
Regulation
[edit]The U.S. Institute of Medicine (IOM) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for minerals in 2001. For manganese, there was not sufficient information to set EARs and RDAs, so needs are described as estimates for Adequate Intakes (AIs). As for safety, the IOM sets Tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. In the case of manganese, the adult UL is set at 11 mg/day. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes (DRIs).[102] Manganese deficiency is rare.[103]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL are defined the same as in the United States. For people ages 15 and older, the AI is set at 3.0 mg/day. AIs for pregnancy and lactation is 3.0 mg/day. For children ages 1–14 years, the AIs increase with age from 0.5 to 2.0 mg/day. The adult AIs are higher than the U.S. RDAs.[104] The EFSA reviewed the same safety question and decided that there was insufficient information to set a UL.[105]
For U.S. food and dietary supplement labeling purposes, the amount in a serving is expressed as a percent of Daily Value (%DV). For manganese labeling purposes, 100% of the Daily Value was 2.0 mg, but as of 27 May 2016 it was revised to 2.3 mg to bring it into agreement with the RDA.[106][107] A table of the old and new adult daily values is provided at Reference Daily Intake.
Excessive exposure or intake may lead to a condition known as manganism, a neurodegenerative disorder that causes dopaminergic neuronal death and symptoms similar to Parkinson's disease.[9][108]
Deficiency
[edit]Manganese deficiency in humans, which is rare, results in a number of medical problems. A deficiency of manganese causes skeletal deformation in animals and inhibits the production of collagen in wound healing.[109]
Exposure
[edit]In water
[edit]Waterborne manganese has a greater bioavailability than dietary manganese. According to results from a 2010 study,[110] higher levels of exposure to manganese in drinking water are associated with increased intellectual impairment and reduced intelligence quotients in school-age children. It is hypothesized that long-term exposure due to inhaling the naturally occurring manganese in shower water puts up to 8.7 million Americans at risk.[111] However, data indicates that the human body can recover from certain adverse effects of overexposure to manganese if the exposure is stopped and the body can clear the excess.[112]
Mn levels can increase in seawater when hypoxic periods occur.[113] Since 1990 there have been reports of Mn accumulation in marine organisms including fish, crustaceans, mollusks, and echinoderms. Specific tissues are targets in different species, including the gills, brain, blood, kidney, and liver/hepatopancreas. Physiological effects have been reported in these species. Mn can affect the renewal of immunocytes and their functionality, such as phagocytosis and activation of pro-phenoloxidase, suppressing the organisms' immune systems. This causes the organisms to be more susceptible to infections. As climate change occurs, pathogen distributions increase, and in order for organisms to survive and defend themselves against these pathogens, they need a healthy, strong immune system. If their systems are compromised from high Mn levels, they will not be able to fight off these pathogens and die.[69]
Gasoline
[edit]
Methylcyclopentadienyl manganese tricarbonyl (MMT) is an additive developed to replace lead compounds for gasolines to improve the octane rating. MMT is used only in a few countries. Fuels containing manganese tend to form manganese carbides, which damage exhaust valves.
Air
[edit]Compared to 1953, levels of manganese in air have dropped.[114] Generally, exposure to ambient Mn air concentrations in excess of 5 μg Mn/m3 can lead to Mn-induced symptoms. Increased ferroportin protein expression in human embryonic kidney (HEK293) cells is associated with decreased intracellular Mn concentration and attenuated cytotoxicity, characterized by the reversal of Mn-reduced glutamate uptake and diminished lactate dehydrogenase leakage.[115]
Regulation
[edit]Manganese exposure in United States is regulated by the Occupational Safety and Health Administration (OSHA).[116] People can be exposed to manganese in the workplace by breathing it in or swallowing it. OSHA has set the legal limit (permissible exposure limit) for manganese exposure in the workplace as 5 mg/m3 over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 1 mg/m3 over an 8-hour workday and a short term limit of 3 mg/m3. At levels of 500 mg/m3, manganese is immediately dangerous to life and health.[117]
Health and safety
[edit]Manganese is essential for human health, albeit in milligram amounts.
The current maximum safe concentration under U.S. EPA rules is 50 μg Mn/L.[118]
Manganism
[edit]Manganese overexposure is most frequently associated with manganism, a rare neurological disorder associated with excessive manganese ingestion or inhalation. Historically, persons employed in the production or processing of manganese alloys[119][120] have been at risk for developing manganism; however, health and safety regulations protect workers in developed nations.[116] The disorder was first described in 1837 by British academic John Couper, who studied two patients who were manganese grinders.[50]
Manganism is a biphasic disorder. In its early stages, an intoxicated person may experience depression, mood swings, compulsive behaviors, and psychosis. Early neurological symptoms give way to late-stage manganism, which resembles Parkinson's disease. Symptoms include weakness, monotone and slowed speech, an expressionless face, tremor, forward-leaning gait, inability to walk backwards without falling, rigidity, and general problems with dexterity, gait and balance.[50][121] Unlike Parkinson's disease, manganism is not associated with loss of the sense of smell and patients are typically unresponsive to treatment with L-DOPA.[122] Symptoms of late-stage manganism become more severe over time even if the source of exposure is removed and brain manganese levels return to normal.[121]
Chronic manganese exposure has been shown to produce a parkinsonism-like illness characterized by movement abnormalities.[123] This condition is not responsive to typical therapies used in the treatment of PD, suggesting an alternative pathway to the typical dopaminergic loss within the substantia nigra.[123] Manganese may accumulate in the basal ganglia, leading to the abnormal movements.[124] A mutation of the SLC30A10 gene, a manganese efflux transporter necessary for decreasing intracellular Mn, has been linked with the development of this Parkinsonism-like disease.[125] The Lewy bodies typical to PD are not seen in Mn-induced parkinsonism.[124]
Animal experiments have given the opportunity to examine the consequences of manganese overexposure under controlled conditions. In (non-aggressive) rats, manganese induces mouse-killing behavior.[126]
Toxicity
[edit]| Hazards | |
|---|---|
| GHS labelling: | |
| H401 | |
| P273, P501[127] | |
| NFPA 704 (fire diamond) | |
Manganese compounds are less toxic than those of other widespread metals, such as nickel and copper.[128] However, exposure to manganese dusts and fumes should not exceed the ceiling value of 5 mg/m3 even for short periods because of its toxicity level.[129] Manganese poisoning has been linked to impaired motor skills and cognitive disorders.[130]
Neurodegenerative diseases
[edit]A protein called DMT1 is the major transporter in manganese absorption from the intestine and may be the major transporter of manganese across the blood–brain barrier. DMT1 also transports inhaled manganese across the nasal epithelium. The proposed mechanism for manganese toxicity is that dysregulation leads to oxidative stress, mitochondrial dysfunction, glutamate-mediated excitotoxicity, and aggregation of proteins.[131]
See also
[edit]- Manganese exporter, membrane transport protein
- List of countries by manganese production
- Parkerizing
References
[edit]- ^ "Standard Atomic Weights: Manganese". CIAAW. 2017.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ a b c d e f g h Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ Mn(–2) is known in Mn(cod)2−2; see John E. Ellis (2006). "Adventures with Substances Containing Metals in Negative Oxidation States". Inorganic Chemistry. 45 (8). doi:10.1021/ic052110i.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ a b Erikson, Keith M.; Ascher, Michael (2019). "Chapter 10. Manganese: Its Role in Disease and Health". In Sigel, Astrid; Freisinger, Eva; Sigel, Roland K. O.; Carver, Peggy L. (eds.). Essential Metals in Medicine:Therapeutic Use and Toxicity of Metal Ions in the Clinic. Metal Ions in Life Sciences. Vol. 19. Berlin: de Gruyter GmbH. pp. 253–266. doi:10.1515/9783110527872-016. ISBN 978-3-11-052691-2. PMID 30855111. S2CID 73725546.
- ^ a b c d e f Emsley, John (2001). "Manganese". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, UK: Oxford University Press. pp. 249–253. ISBN 978-0-19-850340-8.
- ^ Roth, Jerome; Ponzoni, Silvia; Aschner, Michael (2013). "Manganese Homeostasis and Transport". In Banci, Lucia (ed.). Metallomics and the Cell. Metal Ions in Life Sciences. Vol. 12. Springer. pp. 169–201. doi:10.1007/978-94-007-5561-1_6. ISBN 978-94-007-5560-4. PMC 6542352. PMID 23595673. Electronic-book ISBN 978-94-007-5561-1.
- ^ a b c d Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). "Mangan". Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 1110–1117. ISBN 978-3-11-007511-3.
- ^ Lide, David R. (2004). Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics. CRC press. ISBN 978-0-8493-0485-9. Archived from the original on 17 December 2019. Retrieved 7 September 2019.
- ^ Manganese at the Encyclopædia Britannica
- ^ a b Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- ^ Clery, Daniel (4 June 2020). "The galaxy's brightest explosions go nuclear with an unexpected trigger: pairs of dead stars". Science. Retrieved 26 July 2021.
- ^ Schaefer, Jeorg; Faestermann, Thomas; Herzog, Gregory F.; Knie, Klaus; Korschinek, Gunther; Masarik, Jozef; Meier, Astrid; Poutivtsev, Michail; Rugel, Georg; Schlüchter, Christian; Serifiddin, Feride; Winckler, Gisela (2006). "Terrestrial manganese-53 – A new monitor of Earth surface processes". Earth and Planetary Science Letters. 251 (3–4): 334–345. Bibcode:2006E&PSL.251..334S. doi:10.1016/j.epsl.2006.09.016.
- ^
- Birck, J.; Rotaru, M.; Allègre, C. (1999). "53Mn-53Cr evolution of the early solar system". Geochimica et Cosmochimica Acta. 63 (23–24): 4111–4117. Bibcode:1999GeCoA..63.4111B. doi:10.1016/S0016-7037(99)00312-9.
- Lugmair, G.; Shukolyukov, A. (1998). "Early solar system timescales according to 53Mn-53Cr systematics". Geochimica et Cosmochimica Acta. 62 (16): 2863–2886. Bibcode:1998GeCoA..62.2863L. doi:10.1016/S0016-7037(98)00189-6.
- Shukolyukov, Alexander; Lugmair, Günter W. (2000). "On The 53Mn Heterogeneity In The Early Solar System". Space Science Reviews. 92: 225–236. Bibcode:2000SSRv...92..225S. doi:10.1023/A:1005243228503.
- Trinquier, A.; Birck, J.; Allègre, C.; Göpel, C.; Ulfbeck, D. (2008). "53Mn–53Cr systematics of the early Solar System revisited". Geochimica et Cosmochimica Acta. 72 (20): 5146–5163. Bibcode:2008GeCoA..72.5146T. doi:10.1016/j.gca.2008.03.023.
- ^ a b c Young, D.A. (1975). "Phase diagrams of the elements". International Nuclear Information System. LNL: 15. Retrieved 30 January 2023.
- ^ a b Dhananjayan, N.; Banerjee, T. (1969). Crystallographic modifications of manganese and their transformation characteristics. Chapter 1 of: Structure of Electro-Deposited Manganese. CSIR-NML. pp. 3–28.
- ^ Kemmitt, R. D. W.; Peacock, R. D. (1973). The Chemistry of Manganese, Technetium and Rhenium. Pergamon Texts in Inorganic Chemistry. Saint Louis: Elsevier Science. p. 778. ISBN 978-1-4831-3806-0. OCLC 961064866.
- ^ Bradley, A.J.; Thewlis, J. (1927). "The crystal structure of α-manganese". Proceedings of the Royal Society of London, Series A. 115 (771): 456–471. Bibcode:1927RSPSA.115..456B. doi:10.1098/rspa.1927.0103. ISSN 0950-1207.
- ^ Lawson, A. C.; Larson, Allen C.; Aronson, M. C.; et al. (1994). "Magnetic and crystallographic order in α-manganese". J. Appl. Phys. 76 (10): 7049–7051. Bibcode:1994JAP....76.7049L. doi:10.1063/1.358024. ISSN 0021-8979.
- ^ a b Prior, Timothy J; Nguyen-Manh, Duc; Couper, Victoria J; Battle, Peter D (2004). "Ferromagnetism in the beta-manganese structure: Fe1.5Pd0.5Mo3N". Journal of Physics: Condensed Matter. 16 (13): 2273–2281. Bibcode:2004JPCM...16.2273P. doi:10.1088/0953-8984/16/13/008. ISSN 0953-8984. S2CID 250784683.
- ^ Funahashi, S.; Kohara, T. (1984). "Neutron diffuse scattering in β-manganese". J. Appl. Phys. 55 (6): 2048–2050. Bibcode:1984JAP....55.2048F. doi:10.1063/1.333561. ISSN 0021-8979.
- ^ a b c Duschanek, H.; Mohn, P.; Schwarz, K. (1989). "Antiferromagnetic and ferromagnetic gamma-manganese generalisation of the fixed-spin-moment method". Physica B: Condensed Matter. 161 (1–3): 139–142. doi:10.1016/0921-4526(89)90120-8. ISSN 0921-4526.
- ^ Bacon, G E; Cowlam, N (1970). "A study of some alloys of gamma -manganese by neutron diffraction". Journal of Physics C: Solid State Physics. 3 (3): 675–686. Bibcode:1970JPhC....3..675B. doi:10.1088/0022-3719/3/3/023. ISSN 0022-3719.
- ^ "Ch. 20". Shriver and Atkins' Inorganic Chemistry. Oxford University Press. 2010. ISBN 978-0-19-923617-6.
- ^ Luft, J. H. (1956). "Permanganate – a new fixative for electron microscopy". Journal of Biophysical and Biochemical Cytology. 2 (6): 799–802. doi:10.1083/jcb.2.6.799. PMC 2224005. PMID 13398447.
- ^
- Man, Wai-Lun; Lam, William W. Y.; Lau, Tai-Chu (2014). "Reactivity of Nitrido Complexes of Ruthenium(VI), Osmium(VI), and Manganese(V) Bearing Schiff Base and Simple Anionic Ligands". Accounts of Chemical Research. 47 (2): 427–439. doi:10.1021/ar400147y. PMID 24047467.
- Goldberg, David P. (2007). "Corrolazines: New Frontiers in High-Valent Metalloporphyrinoid Stability and Reactivity". Accounts of Chemical Research. 40 (7): 626–634. doi:10.1021/ar700039y. PMID 17580977.
- ^ Greenwood & Earnshaw 1984, pp. 1221–22.
- ^ Heyes, Peter J.; Anastasakis, Konstantinos; De Jong, Wiebren; Van Hoesel, Annelies; Roebroeks, Wil; Soressi, Marie (2016). "Selection and Use of Manganese Dioxide by Neanderthals". Scientific Reports. 6: 22159. Bibcode:2016NatSR...622159H. doi:10.1038/srep22159. PMC 4770591. PMID 26922901.
- ^ Greenwood & Earnshaw 1984, pp. 1218–20.
- ^ Yano, Junko; Yachandra, Vittal (2014). "Mn4Ca Cluster in Photosynthesis: Where and How Water is Oxidized to Dioxygen". Chemical Reviews. 114 (8): 4175–4205. doi:10.1021/cr4004874. PMC 4002066. PMID 24684576.
- ^ Arslan, Evrim; Lalancette, Roger A.; Bernal, Ivan (2017). "An historic and scientific study of the properties of metal(III) tris-acetylacetonates". Struct Chem. 28: 201–212. doi:10.1007/s11224-016-0864-0.
- ^ Rayner-Canham, Geoffrey; Overton, Tina (2003). Descriptive Inorganic Chemistry. Macmillan. p. 491. ISBN 0-7167-4620-4..
- ^ Schmidt, Max (1968). "VII. Nebengruppe". Anorganische Chemie II (in German). Wissenschaftsverlag. pp. 100–109.
- ^ Kadassery, Karthika J.; MacMillan, Samantha N.; Lacy, David C. (2019). "Resurgence of Organomanganese(I) Chemistry. Bidentate Manganese(I) Phosphine–Phenol(ate) Complexes". Inorganic Chemistry. 58 (16): 10527–10535. doi:10.1021/acs.inorgchem.9b00941. PMID 31247867.
- ^ Girolami, Gregory S.; Wilkinson, Geoffrey; Thornton-Pett, Mark; Hursthouse, Michael B. (1983). "Hydrido, alkyl, and ethylene 1,2-bis(dimethylphosphino)ethane complexes of manganese and the crystal structures of MnBr2(dmpe)2, [Mn(AlH4)(dmpe)2]2 and MnMe2(dmpe)2". Journal of the American Chemical Society. 105 (22): 6752–6753. Bibcode:1983JAChS.105.6752G. doi:10.1021/ja00360a054.
- ^ languagehat (28 May 2005). "MAGNET". languagehat.com. Retrieved 18 June 2020.
- ^ Pliny the Elder. "Chapter 25—THE MAGNET: THREE REMEDIES". Natural History of Pliny. BOOK XXXVI. THE NATURAL HISTORY OF STONES.
- ^ Calvert, J. B. (24 January 2003). "Chromium and Manganese". Archived from the original on 31 December 2016. Retrieved 10 December 2022.
- ^ Chalmin, Emilie; Menu, Michel; Vignaud, Colette (2003). "Analysis of rock art painting and technology of Palaeolithic painters". Measurement Science and Technology. 14 (9): 1590–1597. doi:10.1088/0957-0233/14/9/310. S2CID 250842390.
- ^ Chalmin, E.; Vignaud, C.; Salomon, H.; Farges, F.; Susini, J.; Menu, M. (2006). "Minerals discovered in paleolithic black pigments by transmission electron microscopy and micro-X-ray absorption near-edge structure" (PDF). Applied Physics A. 83 (12): 213–218. Bibcode:2006ApPhA..83..213C. doi:10.1007/s00339-006-3510-7. hdl:2268/67458. S2CID 9221234.
- ^ Sayre, E. V.; Smith, R. W. (1961). "Compositional Categories of Ancient Glass". Science. 133 (3467): 1824–1826. Bibcode:1961Sci...133.1824S. doi:10.1126/science.133.3467.1824. PMID 17818999. S2CID 25198686.
- ^ a b c Mccray, W. Patrick (1998). "Glassmaking in renaissance Italy: The innovation of venetian cristallo". JOM. 50 (5): 14–19. Bibcode:1998JOM....50e..14M. doi:10.1007/s11837-998-0024-0. S2CID 111314824.
- ^ Rancke-Madsen, E. (1975). "The Discovery of an Element". Centaurus. 19 (4): 299–313. Bibcode:1975Cent...19..299R. doi:10.1111/j.1600-0498.1975.tb00329.x.
- ^ Miśkowiec, Paweł (2022). "Name game: the naming history of the chemical elements—part 1—from antiquity till the end of 18th century". Foundations of Chemistry. 25: 29–51. doi:10.1007/s10698-022-09448-5.
- ^ Hadfield, Robert (1927). "The metal manganese and its properties: also ores, and the production of ferro-manganese and its history". The Journal of the Iron and Steel Institute. 115 (1): 251–252.
- ^ Alessio, L.; Campagna, M.; Lucchini, R. (2007). "From lead to manganese through mercury: mythology, science, and lessons for prevention". American Journal of Industrial Medicine. 50 (11): 779–787. doi:10.1002/ajim.20524. PMID 17918211.
- ^ a b c Couper, John (1837). "On the effects of black oxide of manganese when inhaled into the lungs". Br. Ann. Med. Pharm. Vital Stat. Gen. Sci. 1: 41–42.
- ^ Olsen, Sverre E.; Tangstad, Merete; Lindstad, Tor (2007). "History of omanganese". Production of Manganese Ferroalloys. Tapir Academic Press. pp. 11–12. ISBN 978-82-519-2191-6.
- ^ a b Preisler, Eberhard (1980). "Moderne Verfahren der Großchemie: Braunstein". Chemie in unserer Zeit (in German). 14 (5): 137–148. doi:10.1002/ciuz.19800140502.
- ^ a b c d Mineral Commodity Summaries 2009 (Report). Water Resources Division, U.S. Geological Survey. 2009. doi:10.3133/mineral2009.
- ^ Bhattacharyya, P. K.; Dasgupta, Somnath; Fukuoka, M.; Roy Supriya (1984). "Geochemistry of braunite and associated phases in metamorphosed non-calcareous manganese ores of India". Contributions to Mineralogy and Petrology. 87 (1): 65–71. Bibcode:1984CoMP...87...65B. doi:10.1007/BF00371403. S2CID 129495326.
- ^ Cook, Nigel J.; Ciobanu, Cristiana L.; Pring, Allan; Skinner, William; Shimizu, Masaaki; Danyushevsky, Leonid; Saini-Eidukat, Bernhardt; Melcher, Frank (2009). "Trace and minor elements in sphalerite: A LA-ICPMS study". Geochimica et Cosmochimica Acta. 73 (16): 4761–4791. Bibcode:2009GeCoA..73.4761C. doi:10.1016/j.gca.2009.05.045.
- ^ Wang, X; Schröder, HC; Wiens, M; Schlossmacher, U; Müller, WEG (2009). "Manganese/polymetallic nodules: micro-structural characterization of exolithobiontic- and endolithobiontic microbial biofilms by scanning electron microscopy". Micron. 40 (3): 350–358. doi:10.1016/j.micron.2008.10.005. PMID 19027306.
- ^ United Nations (1978). "Manganese Nodules: Dimensions and Perspectives". Marine Geology. Natural Resources Forum Library. 41 (3–4). Springer: 343. Bibcode:1981MGeol..41..343C. doi:10.1016/0025-3227(81)90092-X. ISBN 978-90-277-0500-6. OCLC 4515098.
- ^ "Manganese Mining in South Africa – Overview". MBendi Information Services. Archived from the original on 5 February 2016. Retrieved 10 December 2022.
- ^ Elliott, R; Coley, K; Mostaghel, S; Barati, M (2018). "Review of Manganese Processing for Production of TRIP/TWIP Steels, Part 1: Current Practice and Processing Fundamentals". JOM. 70 (5): 680–690. Bibcode:2018JOM....70e.680E. doi:10.1007/s11837-018-2769-4. S2CID 139950857.
- ^ Corathers, L. A.; Machamer, J. F. (2006). "Manganese". Industrial Minerals & Rocks: Commodities, Markets, and Uses (7th ed.). SME. pp. 631–636. ISBN 978-0-87335-233-8.
- ^ a b Zhang, Wensheng; Cheng, Chu Yong (2007). "Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide". Hydrometallurgy. 89 (3–4): 137–159. Bibcode:2007HydMe..89..137Z. doi:10.1016/j.hydromet.2007.08.010.
- ^ Chow, Norman; Nacu, Anca; Warkentin, Doug; Aksenov, Igor & Teh, Hoe (2010). "The Recovery of Manganese from low grade resources: bench scale metallurgical test program completed" (PDF). Kemetco Research Inc. Archived from the original (PDF) on 2 February 2012.
- ^ "The CIA secret on the ocean floor". BBC News. 19 February 2018. Retrieved 3 May 2018.
- ^ "Project Azorian: The CIA's Declassified History of the Glomar Explorer". National Security Archive at George Washington University. 12 February 2010. Retrieved 18 September 2013.
- ^ Hein, James R. (January 2016). Encyclopedia of Marine Geosciences - Manganese Nodules. Springer. pp. 408–412. Retrieved 2 February 2021.
- ^ International Seabed Authority. "Polymetallic Nodules" (PDF). isa.org. International Seabed Authority. Archived from the original (PDF) on 23 October 2021. Retrieved 2 February 2021.
- ^ Oebius, Horst U; Becker, Hermann J; Rolinski, Susanne; Jankowski, Jacek A (January 2001). "Parametrization and evaluation of marine environmental impacts produced by deep-sea manganese nodule mining". Deep Sea Research Part II: Topical Studies in Oceanography. 48 (17–18): 3453–3467. Bibcode:2001DSRII..48.3453O. doi:10.1016/s0967-0645(01)00052-2. ISSN 0967-0645.
- ^ Thompson, Kirsten F.; Miller, Kathryn A.; Currie, Duncan; Johnston, Paul; Santillo, David (2018). "Seabed Mining and Approaches to Governance of the Deep Seabed". Frontiers in Marine Science. 5. doi:10.3389/fmars.2018.00480. hdl:10871/130176. S2CID 54465407.
- ^ a b Hernroth, Bodil; Tassidis, Helena; Baden, Susanne P. (March 2020). "Immunosuppression of aquatic organisms exposed to elevated levels of manganese: From global to molecular perspective". Developmental & Comparative Immunology. 104: 103536. doi:10.1016/j.dci.2019.103536. ISSN 0145-305X. PMID 31705914. S2CID 207935992.
- ^ Ray, Durbar; Babu, E. V. S. S. K.; Surya Prakash, L. (1 January 2017). "Nature of Suspended Particles in Hydrothermal Plume at 3°40'N Carlsberg Ridge:A Comparison with Deep Oceanic Suspended Matter". Current Science. 112 (1): 139. doi:10.18520/cs/v112/i01/139-146. ISSN 0011-3891.
- ^ a b Sim, Nari; Orians, Kristin J. (October 2019). "Annual variability of dissolved manganese in Northeast Pacific along Line-P: 2010–2013". Marine Chemistry. 216: 103702. Bibcode:2019MarCh.21603702S. doi:10.1016/j.marchem.2019.103702. ISSN 0304-4203. S2CID 203151735.
- ^ Bartlett, Richmond; Ross, Donald (2005). "Chemistry of Redox Processes in Soils". In Tabatabai, M.A.; Sparks, D.L. (eds.). Chemical Processes in Soils. SSSA Book Series, no. 8. Madison, Wisconsin: Soil Science Society of America. pp. 461–487. LCCN 2005924447.
- ^ Dixon, Joe B.; White, G. Norman (2002). "Manganese Oxides". In Dixon, J.B.; Schulze, D.G. (eds.). Soil Mineralogy with Environmental Applications. SSSA Book Series no. 7. Madison, Wisconsin: Soil Science Society of America. pp. 367–386. LCCN 2002100258.
- ^ Verhoeven, John D. (2007). Steel metallurgy for the non-metallurgist. Materials Park, Ohio: ASM International. pp. 56–57. ISBN 978-0-87170-858-8.
- ^ Manganese USGS 2006
- ^ Dastur, Y. N.; Leslie, W. C. (1981). "Mechanism of work hardening in Hadfield manganese steel". Metallurgical Transactions A. 12 (5): 749–759. Bibcode:1981MTA....12..749D. doi:10.1007/BF02648339. S2CID 136550117.
- ^ Stansbie, John Henry (2007). Iron and Steel. Read Books. pp. 351–352. ISBN 978-1-4086-2616-0.
- ^ Brady, George S.; Clauser, Henry R.; Vaccari. John A. (2002). Materials Handbook: an encyclopedia for managers, technical professionals, purchasing and production managers, technicians, and supervisors. New York, NY: McGraw-Hill. pp. 585–587. ISBN 978-0-07-136076-0.
- ^ Tweedale, Geoffrey (1985). "Sir Robert Abbott Hadfield F.R.S. (1858–1940), and the Discovery of Manganese Steel Geoffrey Tweedale". Notes and Records of the Royal Society of London. 40 (1): 63–74. doi:10.1098/rsnr.1985.0004. JSTOR 531536. S2CID 73176861.
- ^ "Chemical properties of 2024 aluminum allow". Metal Suppliers Online, LLC. Retrieved 30 April 2009.
- ^ a b Kaufman, John Gilbert (2000). "Applications for Aluminium Alloys and Tempers". Introduction to aluminum alloys and tempers. ASM International. pp. 93–94. ISBN 978-0-87170-689-8.
- ^ a b Dell, R. M. (2000). "Batteries fifty years of materials development". Solid State Ionics. 134 (1–2): 139–158. doi:10.1016/S0167-2738(00)00722-0.
- ^ "WSK1216" (PDF). vishay. Vishay Intertechnology. Retrieved 30 April 2022.
- ^ Reidies, Arno H. (2000). "Manganese Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a16_123. ISBN 9783527303854.
- ^ "EPA Comments on the Gasoline Additive MMT". epa.gov. EPA. 5 October 2015. Retrieved 25 June 2023.
- ^ Shorter Oxford English Dictionary (5th ed.). Oxford University Press. 2002. ISBN 978-0-19-860457-0.
A red brown earth containing iron and manganese oxides and darker than ochre and sienna, used to make various pigments.
- ^ Chen, Daquin; Zhou, Yang; Zhong, Jiasong (2016). "A review on Mn4+ activators in solids for warm white light-emitting diodes". RSC Advances. 6 (89): 86285–86296. Bibcode:2016RSCAd...686285C. doi:10.1039/C6RA19584A.
- ^ Baur, Florian; Jüstel, Thomas (2016). "Dependence of the optical properties of Mn4+ activated A2Ge4O9 (A=K,Rb) on temperature and chemical environment". Journal of Luminescence. 177: 354–360. Bibcode:2016JLum..177..354B. doi:10.1016/j.jlumin.2016.04.046.
- ^ Jansen, T.; Gorobez, J.; Kirm, M.; Brik, M. G.; Vielhauer, S.; Oja, M.; Khaidukov, N. M.; Makhov, V. N.; Jüstel, T. (1 January 2018). "Narrow Band Deep Red Photoluminescence of Y2Mg3Ge3O12:Mn4+,Li+ Inverse Garnet for High Power Phosphor Converted LEDs". ECS Journal of Solid State Science and Technology. 7 (1): R3086 – R3092. doi:10.1149/2.0121801jss. S2CID 103724310.
- ^ Jansen, Thomas; Baur, Florian; Jüstel, Thomas (2017). "Red emitting K2NbF7:Mn4+ and K2TaF7:Mn4+ for warm-white LED applications". Journal of Luminescence. 192: 644–652. Bibcode:2017JLum..192..644J. doi:10.1016/j.jlumin.2017.07.061.
- ^ Zhou, Zhi; Zhou, Nan; Xia, Mao; Yokoyama, Meiso; Hintzen, H. T. (Bert) (6 October 2016). "Research progress and application prospects of transition metal Mn4+-activated luminescent materials". Journal of Materials Chemistry C. 4 (39): 9143–9161. doi:10.1039/c6tc02496c.
- ^ "TriGain LED phosphor system using red Mn4+-doped complex fluorides" (PDF). GE Global Research. Retrieved 10 December 2022.
- ^ Kuwahara, Raymond T.; Skinner III, Robert B.; Skinner Jr., Robert B. (2001). "Nickel coinage in the United States". Western Journal of Medicine. 175 (2): 112–114. doi:10.1136/ewjm.175.2.112. PMC 1071501. PMID 11483555.
- ^ "Design of the Sacagawea dollar". United States Mint. Archived from the original on 22 April 2021. Retrieved 4 May 2009.
- ^ Shepard, Anna Osler (1956). "Manganese and Iron–Manganese Paints". Ceramics for the Archaeologist. Carnegie Institution of Washington. pp. 40–42. ISBN 978-0-87279-620-1.
- ^ Li, Jun; Lorger, Simon; Stalick, Judith K.; Sleight, Arthur W.; Subramanian, M. A. (3 October 2016). "From Serendipity to Rational Design: Tuning the Blue Trigonal Bipyramidal Mn 3+ Chromophore to Violet and Purple through Application of Chemical Pressure". Inorganic Chemistry. 55 (19): 9798–9804. doi:10.1021/acs.inorgchem.6b01639. ISSN 0020-1669. PMID 27622607.
- ^ Silverman, Elian (28 June 2018). "How on earth do you discover a brand-new blue pigment? By accident". TED Ideas. Retrieved 26 June 2024.
- ^ Rice, Derek B.; Massie, Allyssa A.; Jackson, Timothy A. (2017). "Manganese–Oxygen Intermediates in O–O Bond Activation and Hydrogen-Atom Transfer Reactions". Accounts of Chemical Research. 50 (11): 2706–2717. doi:10.1021/acs.accounts.7b00343. PMID 29064667.
- ^ Umena, Yasufumi; Kawakami, Keisuke; Shen, Jian-Ren; Kamiya, Nobuo (May 2011). "Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å" (PDF). Nature. 473 (7345): 55–60. Bibcode:2011Natur.473...55U. doi:10.1038/nature09913. PMID 21499260. S2CID 205224374.
- ^ Dismukes, G. Charles; Willigen, Rogier T. van (2006). "Manganese: The Oxygen-Evolving Complex & Models". Manganese: The Oxygen-Evolving Complex & Models Based in part on the article Manganese: Oxygen-Evolving Complex & Models by Lars-Erik Andréasson & Tore Vänngård which appeared in the Encyclopedia of Inorganic Chemistry, First Edition, First Edition. Encyclopedia of Inorganic Chemistry. doi:10.1002/0470862106.ia128. ISBN 978-0470860786.
- ^ Takeda, A. (2003). "Manganese action in brain function". Brain Research Reviews. 41 (1): 79–87. doi:10.1016/S0165-0173(02)00234-5. PMID 12505649. S2CID 1922613.
- ^ a b Institute of Medicine (US) Panel on Micronutrients (2001). "Manganese". Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Chromium, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Chromium. National Academy Press. pp. 394–419. ISBN 978-0-309-07279-3. PMID 25057538.
- ^ See "Manganese". Micronutrient Information Center. Oregon State University Linus Pauling Institute. 23 April 2014.
- ^ "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017.
- ^ Tolerable Upper Intake Levels For Vitamins And Minerals (PDF), European Food Safety Authority, 2006
- ^ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF).
- ^ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- ^ Silva Avila, Daiana; Luiz Puntel, Robson; Aschner, Michael (2013). "Manganese in Health and Disease". In Astrid Sigel; Helmut Sigel; Roland K. O. Sigel (eds.). Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. Vol. 13. Springer. pp. 199–227. doi:10.1007/978-94-007-7500-8_7. ISBN 978-94-007-7499-5. PMC 6589086. PMID 24470093.
- ^ Wang, Cui-Yue; Xia, Wei-Hao; Wang, Lin; Wang, Zhen-Yong (1 November 2021). "Manganese deficiency induces avian tibial dyschondroplasia by inhibiting chondrocyte proliferation and differentiation". Research in Veterinary Science. 140: 164–170. doi:10.1016/j.rvsc.2021.08.018. PMID 34481207.
- ^ Bouchard, M. F; Sauvé, S; Barbeau, B; Legrand, M; Bouffard, T; Limoges, E; Bellinger, D. C; Mergler, D (2011). "Intellectual impairment in school-age children exposed to manganese from drinking water". Environmental Health Perspectives. 119 (1): 138–143. Bibcode:2011EnvHP.119..138B. doi:10.1289/ehp.1002321. PMC 3018493. PMID 20855239.
- ^ Barceloux, Donald; Barceloux, Donald (1999). "Manganese". Clinical Toxicology. 37 (2): 293–307. doi:10.1081/CLT-100102427. PMID 10382563.
- ^ Devenyi, A. G; Barron, T. F; Mamourian, A. C (1994). "Dystonia, hyperintense basal ganglia, and high whole blood manganese levels in Alagille's syndrome". Gastroenterology. 106 (4): 1068–71. doi:10.1016/0016-5085(94)90769-2. PMID 8143974. S2CID 2711273.
- ^ Hernroth, Bodil; Krång, Anna-Sara; Baden, Susanne (February 2015). "Bacteriostatic suppression in Norway lobster (Nephrops norvegicus) exposed to manganese or hypoxia under pressure of ocean acidification". Aquatic Toxicology. 159: 217–224. Bibcode:2015AqTox.159..217H. doi:10.1016/j.aquatox.2014.11.025. ISSN 0166-445X. PMID 25553539.
- ^ Agency for Toxic Substances and Disease Registry (2012) 6. Potential for human exposure, in Toxicological Profile for Manganese, Atlanta, GA: U.S. Department of Health and Human Services.
- ^ Yin, Z.; Jiang, H.; Lee, E. S.; Ni, M.; Erikson, K. M.; Milatovic, D.; Bowman, A. B.; Aschner, M. (2010). "Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation" (PDF). Journal of Neurochemistry. 112 (5): 1190–8. doi:10.1111/j.1471-4159.2009.06534.x. PMC 2819584. PMID 20002294.
- ^ a b "Safety and Health Topics: Manganese Compounds (as Mn)". U.S. Occupational Safety and Health Administration.
- ^ "NIOSH Pocket Guide to Chemical Hazards – Manganese compounds and fume (as Mn)". Centers for Disease Control. Retrieved 19 November 2015.
- ^ "Drinking Water Contaminants". US EPA. Retrieved 2 February 2015.
- ^ Baselt, R. (2008) Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, pp. 883–886, ISBN 0-9626523-7-7.
- ^ Normandin, Louise; Hazell, A. S. (2002). "Manganese neurotoxicity: an update of pathophysiologic mechanisms". Metabolic Brain Disease. 17 (4): 375–87. doi:10.1023/A:1021970120965. PMID 12602514. S2CID 23679769.
- ^ a b Cersosimo, M. G.; Koller, W.C. (2007). "The diagnosis of manganese-induced parkinsonism". NeuroToxicology. 27 (3): 340–346. doi:10.1016/j.neuro.2005.10.006. PMID 16325915.
- ^ Lu, C. S.; Huang, C.C; Chu, N.S.; Calne, D.B. (1994). "Levodopa failure in chronic manganism". Neurology. 44 (9): 1600–1602. doi:10.1212/WNL.44.9.1600. PMID 7936281. S2CID 38040913.
- ^ a b Guilarte TR, Gonzales KK (August 2015). "Manganese-Induced Parkinsonism Is Not Idiopathic Parkinson's Disease: Environmental and Genetic Evidence". Toxicological Sciences (Review). 146 (2): 204–12. doi:10.1093/toxsci/kfv099. PMC 4607750. PMID 26220508.
- ^ a b Kwakye GF, Paoliello MM, Mukhopadhyay S, Bowman AB, Aschner M (July 2015). "Manganese-Induced Parkinsonism and Parkinson's Disease: Shared and Distinguishable Features". Int J Environ Res Public Health (Review). 12 (7): 7519–40. doi:10.3390/ijerph120707519. PMC 4515672. PMID 26154659.
- ^ Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M (November 2016). "Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies". BMC Pharmacology & Toxicology (Review). 17 (1): 57. doi:10.1186/s40360-016-0099-0. PMC 5097420. PMID 27814772.
- ^ Lazrishvili, I.; et al. (2016). "Manganese loading induces mouse-killing behaviour in nonaggressive rats". Journal of Biological Physics and Chemistry. 16 (3): 137–141. doi:10.4024/31LA14L.jbpc.16.03.
- ^ "Safety Data Sheet". Sigma-Aldrich. Retrieved 26 July 2021.
- ^ Hasan, Heather (2008). Manganese. The Rosen Publishing Group. p. 31. ISBN 978-1-4042-1408-8.
- ^ "Manganese Chemical Background". Metcalf Institute for Marine and Environmental Reporting University of Rhode Island. April 2006. Archived from the original on 28 August 2006. Retrieved 30 April 2008.
- ^ "Risk Assessment Information System Toxicity Summary for Manganese". Oak Ridge National Laboratory. Retrieved 23 April 2008.
- ^ Prabhakaran, K.; Ghosh, D.; Chapman, G.D.; Gunasekar, P.G. (2008). "Molecular mechanism of manganese exposure-induced dopaminergic toxicity". Brain Research Bulletin. 76 (4): 361–367. doi:10.1016/j.brainresbull.2008.03.004. ISSN 0361-9230. PMID 18502311. S2CID 206339744.
Sources
[edit]- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.



